Copper is an essential micronutrient that plays a vital role in the metabolic processes of plants. This blog will explore the multifaceted functions of copper within plant systems, highlighting its importance in various biochemical and physiological pathways. Readers will understand how copper deficiency or toxicity can impact plant health and how optimal copper levels contribute to robust plant growth. We will also discuss modern agricultural practices for managing copper levels in soil to ensure the health and productivity of crops. This comprehensive look at copper’s role in metabolism aims to provide valuable insights for farmers, horticulturists, and anyone interested in plant science and nutrition.
What is Copper Homeostasis in Plants?
Researching Copper Uptake Mechanisms
Copper uptake is a highly regulated process in plants, which requires the involvement of specific transporter proteins. These transporters are found at the cell membrane of the root cells, which, in this case, are responsible for the uptake of copper from the environment. Upon entering the plant, the copper ions are escorted by specific proteins so that free copper does not injure the cells. These ions are then immobilized in the cells in various compartments, where the ions are used for biochemical reactions like photosynthesis and respiration. Such a precisely regulated system allows plants to achieve homeostasis for copper in cells by regulating copper intake and its requirements for cellular metabolism and avoiding both excess and insolvent copper states.
Importance of Copper Transporters in Plant Cells
Regarding copper transporters located within the plant cell cytoplasm, these proteins are primarily involved in the intracellular copper transport processes and the maintenance of the cell’s cbcellscells. Among these transporters are the ones called COPT (Copper Transporter), which is involved in copper ion acquisition of high affinity at the plasma membrane. In addition, ATPase transporters, particularly the heavy metal ATPases (HMAs), assist in the uptake of the copper ion into organelles active with copper-utilizing enzymes, like the chloroplasts. Similarly, copper chaperones, including CCS (Copper Chaperone for Superoxide Dismutase), facilitate the intracellular delivery of copper to its utilization sites to avoid the generation of destructive free radicals from free copper ions. Hence, this transport system was required and contributed to properly using copper without causing any adverse effects associated with excess copper within the plant cells.
The Role of Cu Concentration for Plant Growth
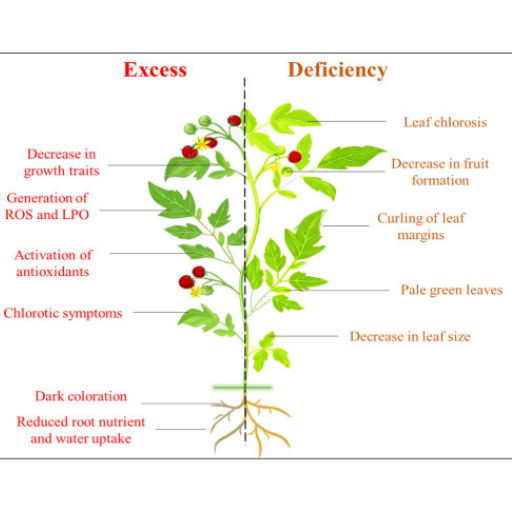
Copper (Cu) concentration is among the most critical requirements for optimal plant growth. Sufficient quantities of copper are necessary for vcriticalphysiological and biochemical activities such as photosynthesis, respiration, lignin formation, and protection from free radical damage. Cu is a helpful ion in several enzymes to improve the reaction rate and sustain better metabolic function. Nevertheless, there is a risk of inadequate copper intake or overconsumption, which can also be harmful. Too little copper causes degenerative changes in anatomy, chlorosis, and troubled development of sexual organs. At the same time, too much copper can lead to excessive free radical generation, thus inhibiting the growth of roots and shoots. Therefore, it is evident that it is essential to maintain a constant copper concentration itoachieve semiproof and well-balanced metabolic processes.
How Does Copper Affect Plant Growth and Development?
Copper Deficiency Physiological Effects In Plants
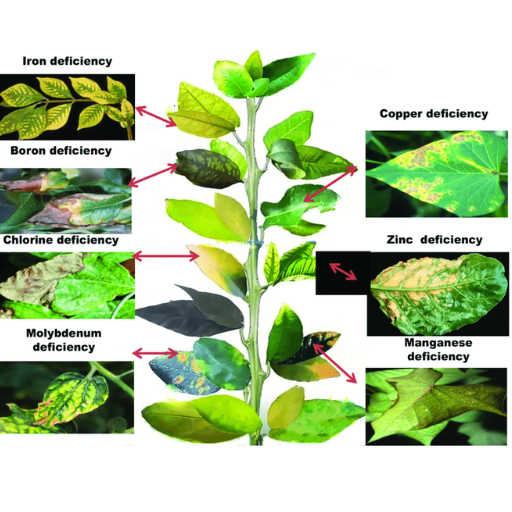
Copper deficiency is apparent in several physiological disturbances of plants. One of the earliest signs of copper deprivation in plants is chlorosis, with the protests mainly occurring in younger leaves as chlorophyll is poorly synthesized. Such a deficit also hinders the photosynthetic process, lowering the energy produced and the growth back. Another process that is also affected is lignin biosynthesis, which enhances the plant’s structural integrity and renders it more resistant to invasion by pathogens. Furthermore, the effect of deficiency on the reproduction of such plants may lead, in some cases, to shallow seed set and seed production in general. Similar depletion is also observed in basic enzyme activities as a number of copper-reliant enzymes are unable to work to their full potential, leading to poor plant growth. Hence, these demonstrate how crucial copper is in plant health and development, for particular physiological abnormalities are averted.
Copper-Induced Stress in Higher Plants
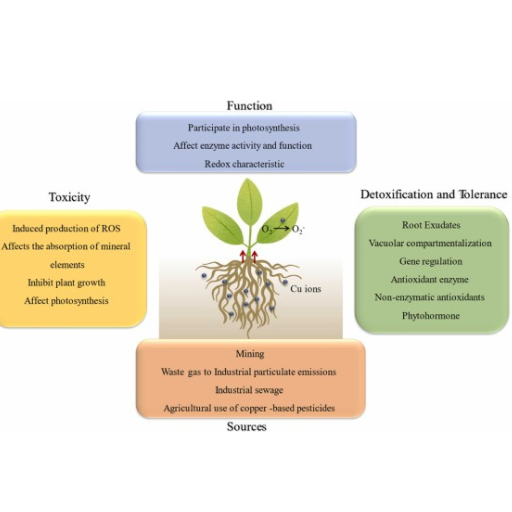
The Plant Stress and Copper Stress Copper-induced stress in higher plants occurs mainly if soil copper concentration is toxic, generating reactive oxygen species (ROS). The ROS species induce oxidative stress, destroying cellular macromolecules such as lipids, proteins, and nucleic acids. This manifests in the cells as signs of stress, including necrosis of dieback of tissues, leaf bleaching, loss of leaf area, and hindering the photosynthesis process. Also, high levels of copper prevent the normal absorption and metabolism of other requisite nutrients, and therefore, normal activities of enzymes and protein synthesis are rendered abnormal. This stress then alters root development thus leaves a plant exposed to drought and lack of nutrients. Hence it is important to care about the soil content of copper to avoid harmful influence and let the plants grow healthy along with the water flows.
Impact of Copper on the Photosynthetic Electron Transport Chain
Too much copper is harmful to the photosynthetic electron transport chain and it is mainly due to the disturbance in chloroplasts that are meant for conducting photosynthesis. Copper at high levels affects the activity of some central elements that comprise the photosystem II (PSII) complex and this affects the photochemistry conversion of the energy from a higher electromagnetic radiation to a lower one in the form of chemical bonds. Copper also interfered with plastocyanin activity, a fundamental electron transport protein that is responsible for the electron relay between PSII and PSI. This then results in the decreased synthesis of ATP and NADPH, which are the molecules required in the Calvin cycle and in the overall metabolism of energy in plants. Thus the damaged electron transport further constrains the photosynthetic activity of the plants and in turn plant growth and the yield of crops are affected.
The Consequences of Copper Toxicity in Plants?

Identification of Cu Toxicity Symptoms
Copper (Cu) toxicity in plants can be expressed via various symptoms, which can be either in stems or in roots. For easily observed symptoms, the initial occurrence of chlorosis, which implies yellowing of leaf tissue as a consequence of leaf tissue with less because chlorophyll is not produced, may be considered. With time, necrosis can take root in the leaves, where leaf tissue dies and turns blackish brown. Other common symptoms include growth retardation in which relatively shorter plant height and poorly formed small leaves[] are generally observed among the affected plants.
In roots, however, copper toxicity forms short solid bodies with limited or no ramification. The tip of the roots can turn brown and die, leading to limited uptake of water and nutrients. Leaf curling, wilting, and leaf discoloration are also standard, in addition to the associated high incidence of pests and diseases. There is a need to maintain the copper level in the soil to the right concentration to prevent and alleviate these detrimental effects on the plants.
Strategies of Plant Bimetallism: Coping with Copper in Plants
Coping with copper stress and sustaining vital activities, endeavors, and functions if challenged are elicited through several goes. One approach is to remove excess copper ions from the free cellular space to non-cytotoxic areas, such as vacuoles. Copper also gets stored within the cells as plants utilize small cysteine-rich proteins called metallothioneins and phytochelatins, which bind copper ions and make them unavailable for bioactivities.
Furthermore, plants resort to oxidative stress protection mechanisms due to the toxic effects of excessive copper. This involves increasing the levels of enzymes such as superoxide dismutase and catalase and peroxidases involved in destroying reactive oxygen species produced during copper stress. Scatter such other adaptation involves the change in the expression of metal transporters for copper’s uptakecopper’stribution within the plant’s tissuesplant’svent accumulation in critical areas.
Finally, it involves the modulation of transduction pathways that activate hormones such as ethylene, abscisic acid, and salicylic acid a balanced response to multi-hormonal redox stress and coordination of physiological and developmental reactions to high copper stress. These mechanisms support the plant in enduring copper stress enabling its survival and growth in an unfavorable environment.
Management Strategies for Control of Excess Copper Content in Soil
To address the issue of excess copper in soil, it is important to integrate both remedial and preventive measures. One of the most frequent practices is the use of organic amendments such as compost and manure or biochar. Such materials may improve the fresh or edible soil’s affordabsoil’sto bind the copper, thus increasing the availability of these nutrients to the soil reducing toxicity risks on the plants.
Another strategy is to modify the soil pH. As copper ions are more soluble in acidic conditions, incorporating lime into the soil to correct its acidic pH also immobilizes and limits the dissolution of copper in the soil and thus its availability. This helps overcome the negative consequences for the plant and also enhances the condition of the soil.
Phytoremediation, which involves planting ex-situ or in-situ plants that can absorb, deaden, and retain copper cations from the surrounding soil, is also a viable practice. Hyperaccumulator plants that thrive and persist in extremely high concentrations of toxic metalliferous are cultivated and harvested so that the amount of copper necessitated in highly polluted soils is progressively lowered.
Lastly, crop rotation and selection of copper-tolerant varieties can be utilized as part of the strategy to correct the soil copper problem. These practices help alleviate the expression of copper toxicity and ensure that agriculture is practiced in a sustainable manner. Implementation of these strategies, either in isolation or in combination, will go a long way in solving the problems and challenges associated with excess copper in the soils.
How Does Copper Modulate Metabolism in Plants?
Crucial Functions of Copper as Complementary Parts of Enzymes
Copper is essential in plants’ physiolplants’acofactorr to several enzymes participating in various metabolic processes. One function of copper is to take part in electron transfer reactions in the electron transport chain, chloroplasts, and mitochondria. This facilitates photosynthesis and respiration, leading to energy generation and carbon fixation.
Copper is also required for metalloenzymes, like cytochrome c oxidase, which is necessary for cell respiration, and superoxide dismutase. This free radical scavenger enzyme detoxifies superoxide radicals by converting them into hydrogen peroxide, a less toxic substance. Other enzymes that need copper, such as polyphenol oxidase and ascorbate oxidase, are alternatively implicated in cell wall fragmentation and reactive oxygen species scavenging, respectively.
The absence or deficiencies of copper add to aberrations in metabolic activities, and symptoms like chlorotic leaves, stunting, and poor stress response are expected. Maintaining copper steady ought to be respected to facilitate enzyme recruitment and for the overall good of a plant.
Relationship Between Copper and Iron in Plant Metabolism
In Plant metabolism, copper and iron share a very intricate and interwoven relationship since both are micro-nutrients. Several biochemical processes require these two trace elements. These two elements are often found within the electron transport chains of mitochondria and chloroplast, which are central to photosynthesis and respiration processes. Copper and iron also function as essential cofactors of different enzymatic molecules, and their levels need to be balanced to optimize the metabolic activities of cells.
Iron binds in structures such as ferredoxin and cytochrome, which are part of the electron transport chain, while copper assists in cytochrome-c-oxidase and plastocyanin. If copper and iron are either excessed or deficient in their concentrations, the metabolic processes in the cell become skewed, leading to excess production of reactive oxygen species and decreased energy production. In addition, the import and long-distance distribution of such metals in plants is subjected to some defined determinants, such as transporters and chelators, to prevent possible toxic levels while guaranteeing a sufficient amount.
An insufficiency or an above-required level of micronutrients can adversely affect the plant. As explained in the previous paragraph, insufficient copper can hinder iron mobilization and -utilization, while excess copper may hinder iron absorption, leading to its deficiency in plants. On the other hand, too much iron may make it difficult for the organisms to absorb copper. Hence, there is a need to achieve a proper balance of copper and iron ion homeostasis to promote normal plant growth and healthy metabolic processes.
Copper’s InflueCopper’slant Nutrient Allocation
Plant and it’s impofor its allocationcation of plant nutrients. It is important tit’se operationit’sllocationnts that are necessary for photosynthesis, respiration, and nitrogen metabolism. Perhaps, one important property of copper is seen in the synthesis of plastocyanin, a polypeptide involved in the electron transport system of plastids and therefore influences the energy synthesis of the plant. Besides, copper has a role in the synthesis of lignin which provides support to the plant by reinforcing the cell walls. Proper copper levels help ensure that these biochemical, structural growth and development processes operate optimally and therefore assist in the general maintenance & distribution of other nutrients. Or limited copper interferes with those processes, the plants will have poor allocation of nutrients, and their growth will be appreciably stunted. At the other extreme, Copper deficiency may occur with other nutrient accumulation, which would be toxic to plants and inhibit the absorption of all other nutrients. Therefore, as with any other minor nutrient, maintaining judicious copper levels plays a vital role in plant nutrient provision and distribution in the plant body.
How Can We Assess Copper Levels in Plant Tissues?

Methods for Measuring Cu Concentration in Plants
To assess copper levels in plant tissues, I typically utilize a combination of laboratory techniques and field tests. One of the most common methods involves using atomic absorption spectroscopy (AAS), which measures the concentration of copper by detecting the light absorbed by the sample. This technique requires the plant tissue to be digested with acids to extract the copper for analysis. Another reliable method is inductively coupled plasma mass spectrometry (ICP-MS), which provides susceptible and precise measurements of copper concentration. Additionally, I sometimes use X-ray fluorescence (XRF) spectroscopy, a non-destructive technique that can quickly determine the copper content by analyzing the emitted secondary X-rays from the sample. Each of these methods has its advantages, and choosing the most appropriate one depends on the specific requirements of the study and available resources.
Interpreting Copper Content Data for Plant Health
When interpreting copper content data for plant health, I first compare the measured levels of copper against established optimal ranges for the specific plant species being studied. These ranges are often determined from scientific research and agricultural guidelines. If the copper concentration falls within the optimum range, it suggests that the plant is likely receiving enough copper for its physiological functions, supporting healthy growth and development.
If the data indicates copper deficiency, I look for symptoms such as stunted growth, chlorosis, or distorted leaves, which can confirm the need for supplementation. Depending on the severity of the deficiency, I might recommend applying copper-containing fertilizers or amending the soil.
Conversely, excessive copper levels could signal potential toxicity, manifesting as leaf discoloration, reduced root growth, or even plant death. To address this, I may advise leaching the soil with water or using chelating agents to bind and reduce soil copper content. By carefully analyzing and responding to copper content data, I strive to maintain the delicate balance of nutrient availability critical for optimal plant health.
Using Copper Chaperones for Plant Stress Management
Using copper chaperones for plant stress management involves leveraging specific proteins that bind and transport copper ions to where they are needed within the plant cells. Copper chaperones help mitigate the adverse effects of both copper deficiency and toxicity. They precisely ferry copper to essential enzymes and proteins that require the metal to function correctly, thus supporting vital physiological processes. By ensuring a balanced distribution of copper, these chaperones help plants better withstand various environmental stresses, such as drought, pathogens, and heavy metal exposure. Effective utilization of copper chaperones can improve plant resilience and overall health.
Reference sources
- Interactions between copper homeostasis and plant metabolism – This source discusses the necessity of copper for plant growth and stress resistance.
- Copper in plants – This article explores the genetic aspects and cellular metabolism requirements of copper in plants.
- Physiological functions of mineral micronutrients – This paper covers the involvement of various micronutrients, including copper, in plant metabolic and cellular functions.
These sources should help validate the feasibility of your topic for readers.
Frequently Asked Questions (FAQs)
Q: What is the significance of high-affinity copper transport in plants?
A: The transport of copper from the environment into a plant and in its organs is mainly achieved by high-affinity copper transport systems. This ensures that in case the availability of Cu is low in the environment, the plants can still take in and utilize the stringent micronutrient in the physiological systems.
Q: To what extent does cu deficiency bring about any adverse effects on the growth and development of plants?
A: Cu deficiency has been implicated in heat stress, stem elongation in teosinte and even suppressed photosynthetic activities in plants among other physiological disorders which affect the maturity of plants. Several metabolic activities require the use of this micronutrient, and when depleted, normal physiological process in plants gets altered.
Q: How are copper levels maintained in Arabidopsis?
A: In Arabidopsis, copper homeostasis has more of a role of nutrients regulation where a number of Cu transporters are involved in the copper uptake, copper distribution and copper storage. These mechanisms aid in the maintenance of copper level within the normal physiological range which is crucial in ensuring both copper deficiency/loss as well as copper excess/toxicity is avoided.
Q: What do fill in the blanks do to plants, when there is excess Cu interment or Cu in the soil?
A: An excess of copper can be detrimental to plants and can cause symptoms such as chlorosis, necrosis, and failure of the root systems to develop in a healthy manner. The individuals deficient in assume the position of radioprotector for several macro and micro macromolecules and ions as well as other substances importante for the plants stems or leaves.
Q: What physiological, genetic or any other changes leads to the enhancement of the cell’s copper stress tolerance?
A: As a result of the low availability of Cu, plants may recruit particular Cu transporters and increase transcription of Cu transport and homeostasis genes. This allows them to cope better with Cu stress and therefore be more efficient in copper use.
Q: What’s the purpose of channelling copper into chloroplasts?
A: The hetero-junction between Cu protodophyllin and photosynthesis induces Fe-Cu in the chloroplasts which are a biochemical centre to conduct photosynthesis. Cu containing proteins also include plastocyanin, an electron carrier in photosynthesis, which further signals that there is a need for adequate Cu supply in plants.






