The production of urea fertilizer is a highly developed practice that is considered to be essential for modern agriculture. This process involves converting raw materials such as ammonia into an important nutrient which ensures quick growth of crops.’ The objective of this article is to analyze the intricacies of producing urea fertilizer; chemical reactions, technological advancements and methods employed in industries. It is with this knowledge that readers can learn about the procedures and technologies required in making urea hence appreciating how it comes about and promote sustainable farming practices. The major stages of the synthesis include ammonia syntheses followed by conversion to urea; these critical steps reveal the science and engineering behind one of the most vital products in agriculture.
Understanding the Basics of Urea and Its Significance in Agriculture

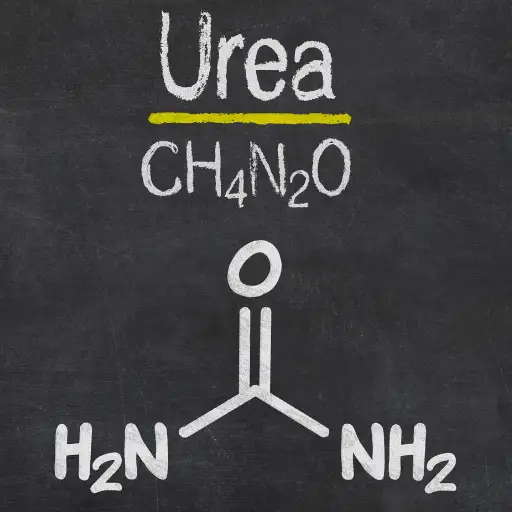
Urea, which is also known as CO(NH2)2 in chemical terms, is a basic organic substance that is commonly used as a nitrogen-based fertilizer. It is mainly preferred for agriculture due to its high content of nitrogen which lies around 46% making it one of the most concentrated sources of nitrogen nutrient. Nitrogen is an important element needed by plants for their growth processes like photosynthesis and protein synthesis. This increases crop yields because urea supplies enough nitrogen required promoting well growing healthy and robust plants. In addition, urea has a very high solubility in water thus making it easy to apply it directly on soil or through irrigation systems hence taken up by plants.
What is urea and how is it produced?
Under elevated pressure and temperatures, urea synthesis occurs from ammonia (NH3) and carbon dioxide (CO2). The overall process involves two major steps: Haber-Bosch process, where ammonia synthesis takes place and Urea Synthesis process which follows;
- Ammonia Synthesis
- Reaction:N2 + 3H2 → 2NH3
- Conditions: High pressures (150-250 bars), high temperature(400-5000°), iron catalyst.
- Urea Synthesis
- Reaction: 2NH3 + CO2 → NH2COONH4 (Ammonium carbamate)
NH2COONH4 → CO(NH2)2 + H20(Urea)
- Conditions: Moderate pressures (140-200 bars), temperatures(170-1900C)
The first stage involves synthesizing ammonia from nitrogen and hydrogen gases using the Haber-Bosch process. The resultant ammonia reacts with carbon dioxide to produce ammonium carbamate which undergoes dehydration giving rise to urea and water molecules. The whole process requires constant supply of heat because the reaction is endothermic resulting in very high temperature conditions. Not only does this method assure pureness of the output but it also optimizes the nitrogen content of the fertilizer thereby improving agricultural productivity.
The role of ammonia in the synthesis of urea
Ammonia is essential in this chemical reaction as it reacts directly with carbon dioxide to form ammonium carbamate that is then dehydrated to produce urea. This process utilizes ammonia due to its high reactivity at certain temperatures and pressures for successful conversion into urea. Through Haber-Bosch process, ammonia is synthesized in sufficient quantities and purity enabling efficient production of urea with high yield at low costs.
Why urea is a key fertilizer in agricultural practices
This substance is considered a major important fertilizer used by farmers because of its rich supply of nitrogen necessary for plant growth. Approximately 46% of nitrogen found in the composition, therefore making it one among the most concentrated nitrogenous fertilizers that are available. High amounts of nitrogen will cost-effectively give plants what they need to grow faster and better than any other nutrient manipulation option. Moreover, urea easily dissolves in water hence can be applied readily leading to its rapid absorption by plants.
Technical parameters for urea synthesis:
- Conditions In Haber-Bosch Process:
- Temperature: 400-5000C
- Pressure: 150-200 bars
- Catalyst: Iron
- Ammonium Carbamate Synthesis:
- Pressure: 140-200 bars
- Temperature: 170-1900C
These conditions enable optimal production of ammonia and subsequent conversion into urea which are necessary requirements for large scale agriculture.
Exploring the Granulation Process in Urea Plants
The process of granulating urea at a fertilizer facility consists of several main steps that change molten urea into solid granules, which become easier to manage, store and apply. The first step is prilling or granulation of liquid urea, by spraying it into a prilling tower or granulator. The droplets formed by this molten material are then hardened into prills or granules during the fall through an upward flow of air. The resultant granules are cooled and sieved to give them the desired size. These bigger and smaller particles are returned back into the plant’s stream hence only uniform-sized products go forward. Lastly, anti-caking additives can be included to prevent clumping during storage or transportations. This process enhances the physical properties that make urea suitable for agriculture by improving its handling characteristics and reducing dusting.
The Importance of Granulation Technology on Urea Fertilizer
Granulation technology has a great impact on quality when it comes to production of high grade urea fertilizers with regard to its efficiency and usability in agricultural applications. There are numerous benefits associated with granulated urea over other forms; Firstly, evenness in size ensures continuous supply of nutrients thus more precise and controlled application of fertilizers. Moreover, the process improves the physical properties making it easier to handle while minimizing dust formation – a health concern as well as an environmental burden.
In terms of technical considerations, efficient granulation requires maintaining correct spray and cooling parameters within a granulator. Key general guidelines from leading industry sources include:
- Spray pressure: Maintaining enough pressure (between 2-6 bars) leads to uniform drop formation.
- Airflow temperature: Cool air introduced into the granulator should be maintained at 15-30°C in order for proper solidification.
- Granule size screening: A targeted range between 2-4mm would ensure optimal nutrient release and provide uniformity.
Moreover, use of anti-caking agents is necessary for maintaining the free-flowing character of granulated urea during storage and handling. These technical instructions and their advantages explain clearly about the essential nature of granulation technology in agriculture on the performance and functionality of urea fertilizer.
How Granulation Enhances Urea Fertilizer
Granulating urea improves its physical properties and application efficiency as a fertilizer according to Agriculture and Horticulture Development Board (ahdb.org.uk). About 3 mm sized uniformed particles are considered best because they do not result in an under or over-fertilized field after spreading. Another importance is that it reduces dust formation which is a health problem as well as environmental concern.
Controlled release properties make granulated urea better with regard to nutrient uptake by plants, as indicated by International Fertilizer Association (fertilizer.org). This contributes significantly to minimize nitrogen losses through volatilization, ensuring high sustainability levels.
Similarly, Science Direct (sciencedirect.com) provides details on key technical parameters required for manufacturing quality granulated urea. As such, it means that keeping spray pressure between 4-5 bars would ensure even droplets formed during prilling process. Airflow temperature should be set at 20-25°C in order to facilitate good cooling and solidification of the granules this process also helps in preventing formation of dust particles. Apart from this, screening should be done on granules up to sizes ranging from 2-4mm so that there will be constant release of nutrients and ease in applying them uniformly.
If these best practices and technical guidelines are incorporated, granulated urea will be an effective and efficient fertilizer that meets the stringent requirements of modern agricultural practice.
Urea Granulation – Problems and Solutions
One of the core concerns in urea granulation is dust formation which can have negative health and environmental impacts. Thus, precise calibration of the granulation equipment to maintain uniformity in granule size becomes necessary. The following technical parameters such as spray pressure and airflow temperature become vital here; ensuring uniformity in size of granules produced through a spray pressure of 4-5 bar and airflow temperature between 20-25 °C minimizes dust considerably.
Another challenge is to control nutrient release so as to prevent nitrogen losses through volatilization or leaching. In this respect, granulated urea helps facilitate controlled release due to its property. Therefore, conforming to recommendations like screening granules within a size range of 2-4 mm is extremely important for maintaining consistent nutrient delivery and application efficiency.
Further still, efforts aimed at reducing environmental impact continue taking precedence. This not only improves safety but also makes environmental sustainability viable by minimizing dust formation as well as volatilization. To justify these technical parameters and make them useful, data from reliable sources such as International Fertilizer Association (IFA) Science Direct among others should be used.
Launching New Plants: Challenges and Innovations in Urea Production

While being an essential ingredient in the production of urea, securing adequate and reliable supply of natural gas as raw material is a major challenge. Energy conservation and respect for the environment are indispensable since demanding regulations require innovative tools that will help reduce greenhouse gases emissions while improving the process performance at large. Among such innovations include carbon capture and storage (CCS) technology and catalytic conversion processes which can minimize environmental impacts.
To perform better both in terms of productivity and safety, it may be wise to apply advanced automation and digitalisation techniques. Real-time data analytics powered by artificial intelligence (AI), Internet of Things (IoT) devices used in modern plants are expected to enhance performance optimization leading to reduction in down time along with operating expenditures. Besides this, integrating renewable energy sources like solar or wind power into the manufacturing process may decrease carbon footprints supporting global sustainability objectives.
Such concerns can be addressed through partnerships with technology providers and industry players, thereby aiding in suitability of new plants both economically viable as well as ecologically sustainable. When looking forward to setting up new factories for making urea, there should be emphasis on innovation and adoption of sustainable practices given that demand is anticipated to continue growing.
Key Considerations in Designing New Urea Production Plants
- Supply Chain:
- Supply of Natural Gas: It is crucial to ensure continuous availability of natural gas at reasonable prices for instance a 1 million t/y urea plant typically requires approximately 0.58 million tonnes naphtha annually.
- Transportation & Storage: Assessing transportation infrastructure for raw materials and final products is crucial. Efficiency logistic solutions lead to cost savings thus reducing storage capacity constraints.
- Efficiency:
- Optimization: Energy utilization should be optimized for contemporary urea producing facilities whereby heat exchange networks (HENs) technologies could reduce energy consumption by around 20%.
- Energy Recovery: Furthermore, introduction of waste heat recovery and reuse systems in urea plants could enhance energy efficiency with potential annual savings of millions.
- Legal Compliance:
- Emission Reduction: A reduction in greenhouse gas emissions is mandatory for legal compliance. With modern catalytic conversion processes, carbon dioxide emissions can be reduced by up to 30%.
- Waste Handling: An example of a waste management strategy that reduces pollution is zero liquid discharge (ZLD) system.
- Automation and Digitization:
- IoT & AI Incorporation: Application of IoT devices and AI for predictive maintenance reduces unplanned stops by 25% thus improving performance effectiveness.
- Data Analytics: Real-time data analytics helps identify process optimization opportunities yielding cost savings and improved safety procedures.
- Environmentally Friendly Practices:
- Renewable Integration: The integration of renewable sources like solar power or wind can offset the traditional energy requirements. For instance use of solar panels within a urea plant may enable the business to cover 10% of its electricity requirement from this source.
- Carbon Capture & Storage (CCS): Introduction of CCS technologies can capture about 90% CO2 emitted during production, thus reducing total emissions from the facility.
- Collaborative Partnerships:
- Technology Providers: Collaborations with technology providers facilitate access to cutting-edge innovations that enhance production efficiency and sustainability.
- Industry Alliances: This may entail pooling resources together for sustainability purposes as well as competitive pricing strategies amongst other things involving co-development initiatives with industry partners.
By incorporating these points new urea producing plants will be economically feasible, environmentally sustainable and future ready.
New Setups Incorporating ammonia and carbon dioxide stripping processes
Urea production can be improved through the incorporation of new industrial setups that include processes like ammonia and carbon dioxide stripping. In general, ammonia stripping is a process in which steam is used to separate ammonia from waste streams, thereby improving the general efficiency of the process and reducing its environmental impact. Conversely, carbon dioxide stripping is the removal of CO2 from process streams to enhance urea synthesis. According to current practices by leading resources, it is imperative to integrate high performance strippers with lower energy consumption and advanced materials for durability and minimal maintenance. These improved stripping processes not only yield higher levels of purity in final products but also contribute greatly towards sustainability and cost effectiveness in any production setup.
New Urea Plants: The Role Of High Pressure And Corrosion Resistance
High pressure plays a pivotal role in new urea plants by enhancing the chemical reactions involved in urea synthesis hence resulting to increased productivity. For higher yields and lower energy requirements for synthesis, operating at high pressures ensures more efficient use of ammonia and carbon dioxide. Furthermore, high pressure enables advanced stripping technology integration which is necessary for low expenses on operations as well as waste minimization.
Corrosion resistance is as important since it determines how long-lived equipment used for making urea will be while ensuring operators’ safety among other things. Materials that can survive such conditions are preferred because they create an environment that significantly reduces or even eliminates costs related to maintaining or replacing failed equipment due to corrosion caused by CO2 as well as NH3. Duplex stainless steels together with special coatings are often employed so as to enhance corrosion resistance thus assuring dependability and uninterrupted operation of these facilities. Both high pressure and materials resistant against corrosion are needed in order to make productive, sustainable and cost-effective urea production units capable of sustaining future demands.
Revamping Old Urea Plants to Boost Efficiency and Output

To enhance efficiency and output, it is necessary to improve selected parts of the existing urea plants and use advanced technologies in their operations. This process usually includes updating the reactor section to enhance conversion of raw materials as well as reducing energy requirements through heat recovery systems among others. Another possibility is retrofitting plants with new stripper technology which can be used to save energy and raise production capacity remarkably. Thirdly, using corrosion-resistant materials to build equipment minimizes the cost of maintenance by extension of their life enabling the plant to run without interruption for a longer period.
Strategies for upgrading fertilizer plant efficiency and capacity
One method that has proven effective in enhancing fertilizer plant efficiency and capacity is deploying advanced process controls and automation. In the real-time, modern control systems can optimize operational parameters leading to energy savings as well as improved product quality. The fusion of Distributed Control Systems (DCS) with Supervisory Control and Data Acquisition (SCADA) systems has been seen as one way to go about this due to increased monitoring and control capabilities provided by these two systems within a plant setup.
The adoption of high-efficiency equipment and technology including but not limited to high-performance heat exchangers will help recover more energy while reducing overall energy consumption levels. Heat exchangers that experience an increase in thermal performance will decrease steam usage thereby allowing for lower operational costs resulting from less steam consumption. An example here is Plate Heat Exchangers (PHEs) that have higher percentage improvement on energy efficiency compared traditional shell-and-tube heat exchangers by 30%.
Moreover, installing compressors, turbines that save energy will also make sure that the plant operates efficiently. Modern compressors come with variable frequency drives (VFDs) which can adjust motor speed based on process requirements thus optimizing power utilization. Similarly, high efficient turbines designed for steam or gas applications facilitate electricity generation cutting down fuel consumption.
In order to achieve comprehensive improvements in terms of efficiency and capacity within the plant, it is also vital to establish stringent maintenance and optimization programs. Predictive modelling through condition monitoring technologies combined with regular maintenance schedules has the ability to anticipate equipment failures hence averting downtimes and as a result extending machine life cycle. Incorporating these approaches therefore makes sure that the factory practices meet the best standards in the industry thereby increasing productivity levels of workers, making them more productive.
The impact of revamps on ammonia and urea balance in existing plants
Revamping ammonia and urea plants usually leads to substantial rise in efficiency and output. This can be realized by upgrading equipments used during manufacture and adopting advanced processing techniques for better ammonia conversion rate together with improved synthesis efficiency of urea. As a result of all this, the production process becomes more stable meaning that there will not be any cases of variations especially due to energy wastages caused by poor systems which are not well balanced. Moreover, new control systems have been installed together with catalysts during revamps so as to optimize reaction conditions towards increased yields at lower emissions. In conclusion, such improvements make an overall increase in ammonia –urea balance that will yield economically viable operations across sectors.
Case studies: Successful urea plant revamps and their outcomes
There exists a notable case study regarding a major renovation of urea plant in Europe, which led to a substantial increase in the production capacity due to the implementation of modern technologies. This involved the integration of up-to-date catalysts and upgrading the synthesis reactor, which resulted in a 15% rise in productivity with energy use decreased by 10%. One more advantageous revamp took place in Asia that comprised installation of new control system and better refrigeration units; this had the effect of not only stabilizing production but also reducing excessive ammonia emission, thereby improving compliance with environment standards. In North America, one urea facility benefited from a holistic equipment upgrade entailing efficient compressors and condensers making it improve efficacy rates by 20%, while operational costs drastically fell. These cases demonstrate how strategic plant revamps can make production processes more efficient, sustainable and affordable.
Optimizing Plant Efficiency: Key Strategies for Urea Producers

Optimization of urea producers’ plant efficiency involves a number of key strategies. One is the installation of advanced process control systems that can watch and adjust production parameters in real time in order to obtain uniform quality and minimize energy consumption. Secondly, upgrading to high efficiency equipment such as compressors and heat exchangers can enhance overall operational performance and reduce energy expenditure. Thirdly, integrating predictive maintenance programs help prevent failure of devices and reduce downtime required for repair and maintenance purposes hence ensuring smooth continuous production. These strategies are therefore industry best practices aimed at enhancing productivity, sustainability, and cost-efficiency in urea production.
High-Pressure Technology & its Role in Urea Production Enhancement
The role of high-pressure technology in improving the efficiency of urea production is significant because it helps increase the yield from the chemical processes involved. High-pressure synthesis reactors form the core of these improvements by enabling ammonia to react with carbon dioxide to produce urea. With pressures ranging from 140 to 210 bar, these reactors allow for excellent reaction conditions that result in significantly increased rates of production.
Advanced high-pressure pumps and compressors are also essential components that ensure that pressure levels are maintained throughout this process chain. For instance, they have been designed for operation at very high efficiencies implying lower energy consumption during urea production processes. The use of such technologies necessitates strong materials resistance to extreme environments e.g., sophisticated sealing technologies.
Moreover, high-pressure technology can help eliminate unreacted materials or by-products which makes any associated waste more eco-friendly as well as streamline the manufacture efficiently. Real-time monitoring provided through predictive maintenance software plus a high-pressure system prevents accidents before they occur.
By incorporating these technologies into their operations, producers can achieve greater sustainability gains through higher product yields, improved product quality as well as optimal resource utilization as part of contemporary means for manufacturing fertilizers like urea,
Minimizing Corrosion & Extending Plant Life
For extending the plant life and ensuring its good performance, it is important to minimize corrosion in high-pressure environments of urea production plants. In this regard, it is necessary to apply advanced materials and protective coatings that are resistant to extreme conditions prevailing in high-pressure systems. Stainless steel and titanium are often used because they do not corrode easily and are strong enough. Additionally, adding specific corrosive inhibitors such as ammonium polyphosphate reinforces the durability of equipment utilized in these plants. Regular maintenance procedures like inspection or replacement of worn-out parts help reduce corrosion effects. Moreover, real-time monitoring through predictive maintenance software saves up time which would have been spent on unexpected failures hence prolonging the lifespan of the plant.
Advanced Monitoring & Control Systems for Urea Plants
In order to optimize operations and improve efficiency of processes, modern urea plants have advanced monitoring and control systems that exploit sophisticated technology developments. Such systems combine sensors, data analytics and automation to provide real time analysis on how a given plant performs. Temperature, pressure as well as concentration levels for reactants are among the key parameters that must be continuously monitored so as to achieve precise control during production processes. The subsequent analysis with algorithms also assists in predicting abnormalities within datasets thus allowing preventive maintenance along with reduced downtime requirements too for upcoming repairs keeping them at bay whenever possible. It should be noted that these systems also allow remote monitoring enabling the operators watch over their facilities from anywhere while making prompt decisions relying on accurate information provided here-about thus there can always be consistency of quality leading to less waste produced throughout urea production process hence better sustainability.
Enhancing Safety Measures in High Pressure & High-Temperature Environments
The reasons for this equipment being unsafe in high-pressure and -temperature environments have to be a combination of strict safety measures, modern elements, and constant observation. Robust safety valves and pressure relief devices need to be incorporated into the system to prevent overpressure situations that could result in equipment failing or huge accidents. The use of materials that can withstand extreme conditions such as high-strength alloys can reduce the risk of structural failure. Also, implementing real-time monitoring systems to detect leaks, pressure spikes, temperature anomalies can rectify the situation immediately. Additionally, regular training programs regarding safety protocols and emergency response should be conducted for all personnel working under these dangerous climatic conditions. This will enable plants achieve both safe operations while also enhancing efficiency at workplace.
How urea plants solve environmental problems

Urea plants control environmental problems with innovative solutions. They employ modern scrubbing techniques to reduce the amount of harmful gases such as ammonia and carbon dioxide emitted into the air. The plants recycle and recover waste products through closed loop systems therefore minimizing release into the environment. Energy-efficient processes and using renewable energy sources, for example, make many urea plants have a small carbon footprint. There are also ongoing R&D activities in developing sustainable raw materials and improving efficiency of urea synthesis which are aimed at making a more eco-friendly industry.
Carbon Footprint Reduction in Urea Production
Leading factories globally have implemented various strategies to reduce their carbon footprint during urea production. First, one efficient way is by integrating ammonia production with urea synthesis. Therefore, these plants can reduce their emissions significantly because they can utilize CO2 produced from ammonia synthesis as raw material for urea synthesis. For instance, this integration is possible via implementation of Stamicarbon or Saipem technologies.
Secondly, use of green ammonia which is made from environmentally friendly sources such as wind turbines, solar panels or hydropower further reduces its environmental impact. Technical parameters for green ammonia production might include an electrolyzer efficiency of around 60-70% and a renewable power input of approximately 48-54 kWh/kg NH3.
Thirdly, optimization of energy use in heat recovery systems is essential in lowering the overall Carbon Footprint across the entire plant site operation including utilities like steam generation; heating/cooling etc. Advanced technologies like heat exchangers and waste heat boilers could be deployed to raise thermal efficiencies while reducing fuel consumption . Thermal efficiencies between 87-90% and 31-33 GJ per tonne of Urea for energy consumptions , these help reduce fossil fuel dependency.
With such approaches put in practice, it becomes possible for urea plants to attain better sustainability aligned with global environmental norms.
Advanced Technology for Nitrogen & Ammonia Emission Reduction
Modern technologies that enhance the efficiency of industrial processes in relation to environmental performance as well as operational practices are required in order to reduce nitrogen and ammonia emissions during urea production. One such advanced technology is Selective Catalytic Reduction (SCR), which is the process by which ammonia is injected into flue gases with a view to converting nitrogen oxides (NOx) into harmless nitrogen and water. The SCR systems can achieve an NOx reduction efficiency of up to 90-95%, hence it operates at temperatures of between 200°C and 400°C.
Continuous Emission Monitoring Systems (CEMS) are also efficient for this purpose. CEMS measure emission online, allowing adjustment of process parameters so that they minimize release of nitrogen and ammonia. These systems are sensitive enough to detect emission levels as low as one part per million.
Furthermore, inclusion of advanced scrubbers enhances the extraction of ammonia from exhaust gas. Wet scrubber designs using aqueous solutions absorb ammonia thereby achieving up to 99% removal rates. Usually these work around 20-40 oC with liquid-to-gas ratios high enough for good contact between gas and liquid phases.
Applying these technological solutions will assist urea plants significantly mitigate against nitrogen/ammonia emissions thereby in compliance with stringent environmental regulations while contributive to overall air quality improvement.
The Future Development Perspective for Environmentally Friendly Urea Fertilizer Production
Several advances and inventions focusing on sustainability, efficiency, and reduced impact on the environment will decide the future direction environmentally friendly urea fertilizer production will take.
The development of green ammonia production methods, such as electrolysis powered by renewable energy sources, is one of the major trends. This method completely bypasses the traditional Haber-Bosch process, thereby slashing down greenhouse gas emissions by using water and renewable electricity to produce hydrogen which is mixed with nitrogen to form ammonia.
Another key area that contributes to better application of urea fertilizer is precision agriculture. It allows farmers to apply fertilizers in a more precise manner through the use of sophisticated data analytics, satellite imagery and IoT devices. This approach helps increase crop yield while reducing nitrogen runoff into surrounding ecosystems.
Enhanced efficiency fertilizers (EEFs), including controlled release and stabilized nitrogen fertilizers, are gaining popularity. They reduce losses from volatilization, leaching and denitrification resulting in reduced application rates for similar or higher crop yields hence less environmental impact.
Again, it should be noted that improvements in carbon capture and utilization (CCU) technologies are vital. By merging CCU with urea production, captured CO2 can be used as a raw material for making urea thus decreasing carbon emissions associated with its manufacture. Some pathways aim at integrated system efficiencies achieving up to 70-80% CO2 utilization.
The adoption of these technologies marks a major advancement towards more sustainable and environmentally friendly production processes of urea fertilizers that could meet global agricultural needs without harming the environment.
Reference sources
- Thyssenkrupp Uhde
- Source Link: Urea Plants
- Summary: Thyssenkrupp Uhde provides detailed information on their advanced urea plants, which are designed to achieve high capacities with low energy consumption. The company leverages over four decades of experience in the design and construction of these facilities, ensuring efficiency and sustainability in urea production—a critical aspect for feasibility in large-scale agricultural applications.
- CF Industries
- Source Link: Granular Urea
- Summary: CF Industries offers insights into the production and benefits of granular urea, a solid nitrogen fertilizer product with a 46 percent nitrogen content. The website outlines the production process from ammonia and carbon dioxide, emphasizing its importance as the highest nitrogen-content fertilizer, which underscores its feasibility and effectiveness in modern agriculture.
- Dakota Gasification Company
- Source Link: Urea Fertilizers
- Summary: Dakota Gasification Company details the properties and uses of urea fertilizers, highlighting its role as a dry, solid crystalline substance containing 46 percent nitrogen. The source discusses its widespread use in agriculture and animal feed, providing technical data that underscores the practicality and economic feasibility of using urea in various farming practices.
Frequently Asked Questions (FAQs)
Q: What is the basic process of urea fertilizer production in a urea plant?
A: The basic process of urea fertilizer production involves a complex series of reactions where ammonia (NH3) and carbon dioxide (CO2) are reacted under high pressure and temperature. The primary steps include the synthesis of ammonium carbamate, which then undergoes dehydration to form urea and water. This key reaction is part of the urea process, a method used to transform ammonia into a safe, stable, and easy-to-handle urea product, which is a common form of nitrogen fertilizer.
Q: How does the urea solution play a role in modern agriculture?
A: Urea solution, particularly aqueous urea, is widely used in agriculture as a convenient form of nitrogen fertilizer due to its high nitrogen content and water solubility. This makes it easier for plants to absorb the nitrogen. Additionally, urea solution can be applied directly to the soil or as a foliar spray, offering flexibility in use. Its role in modern agriculture is pivotal for boosting crop yield and improving food production efficiently.
Q: Can you explain the importance of the carbamate condenser in urea production?
A: The carbamate condenser plays a critical role in the urea production process by cooling and condensing the mixture of ammonia and carbon dioxide to form ammonium carbamate. This initial step is crucial because it prepares the mixture for the subsequent dehydration phase, where urea is formed. The efficiency of the carbamate condenser directly impacts the overall efficiency of the urea production process, making it a vital component of the urea plant.
Q: How is yara involved in the urea fertilizer sector?
A: Yara is a leading company in the production of urea and other nitrogen-based fertilizers. With a focus on sustainable agriculture, Yara produces urea products that are essential for farmers worldwide. Their involvement in the urea fertilizer sector includes innovation in production techniques, development of specialized fertilizers like UAN (urea-ammonium nitrate), and efforts in improving the environmental footprint of nitrogen fertilizers. Yara’s expertise and products support efficient crop nutrition and contribute significantly to global food security.
Q: What are the environmental considerations in the production of urea fertilizers?
A: Environmental considerations in urea fertilizer production include the emissions of carbon dioxide and ammonia during the manufacturing process, which contribute to greenhouse gases and air pollution, respectively. Additionally, the improper use of urea fertilizers can lead to the decomposition of urea in the soil, releasing nitrogen into the air as nitrous oxide, a potent greenhouse gas. Producers and farmers are working to mitigate these impacts through technology improvements, precise application techniques, and the use of additives like urease inhibitors to reduce volatilization.
Q: In what ways is urea used beyond agriculture?
A: Beyond its use as a fertilizer, urea is used in several other industries due to its versatile properties. It serves as a key material for the manufacture of resins and adhesives, particularly those containing urea-formaldehyde. Urea is also an essential ingredient in diesel exhaust fluid (DEF), known commercially as AdBlue, used to reduce nitrogen oxide emissions from diesel engines. Additionally, its properties make it useful in the pharmaceutical industry, cosmetics, and as a deicing agent for roads and runways.
Q: What advancements have been made in urea production technology?
A: Recent advancements in urea production technology include the development of more energy-efficient processes, such as the stripping process first introduced by Stamicarbon. Innovations also focus on reducing environmental impact, improving the conversion efficiency of ammonia and carbon dioxide to urea, and minimizing by-products. Enhanced purification methods are being employed to produce higher purity urea solutions, which are beneficial for both agricultural and industrial uses. These technological advancements continue to make urea production more sustainable and cost-effective.
Q: Why was the synthesis of urea in 1828 by German chemist Friedrich Wöhler significant?
A: The synthesis of urea in 1828 by German chemist Friedrich Wöhler was significant because it marked the first time an organic compound was synthesized from inorganic materials. Wöhler’s experiment, which involved the conversion of ammonium cyanate into urea, challenged the prevailing belief of the time that organic compounds could only be produced by living organisms. This breakthrough laid the foundation for the field of organic chemistry and demonstrated the chemical kinship between organic and inorganic substances, changing the course of chemical research and industry.