All living beings crucially require nitrogen for growth and development of plants. Nitrogen is the key component in forming amino acids, proteins and nucleic acids that are essential for organic processes that keep living organisms alive. The second most important function of nitrogen is in producing chlorophyll in plants, which is required for photosynthesis. In this process, sunlight gets converted to chemical energy used by plants for growth and survival.
This blog intends to discuss the many aspects of how nitrogen benefits plant life by exploring their physiological and biochemical functions. Also we will consider how access to nitrogen affects crops’ health, productivity, and resilience and address soil nitrogen management practices associated with desirable agricultural outcomes. By realizing how crucial it is, farmers will be able to understand sustainable farming methods among other things about nitrogen cycling within ecosystems.
What is the Role of Nitrogen in Plant Growth and Development?

Plants need nitrogen to function because it is a fundamental nutrient that helps in carrying out various key physiological processes. It also forms part of chlorophyll, the molecule that conducts photosynthesis, allowing plants to develop carbohydrates from sunlight, water, and carbon dioxide. Also, nitrogen forms amino acids that are used in constructing proteins, enzymes, and coenzymes involved in crucial biochemical reactions. Nitrogen within nucleic acids is necessary as DNA and RNA provide important roles in cell division and growth. As such an adequate supply of nitrogen has direct effect on improved vegetative growth, enhanced protein synthesis and general plant vigor. When there is insufficient nitrogen available, plants fail to attain optimal metabolic activity leading to retarded growth, yellowing leaves (chlorosis), and reduced yield.
How Do Plants Use Nitrogen?
The major way through which plants obtain Nitrogen is through their root systems whereby it can be taken up as nitrate (NO3-) or ammonium (NH4+). Nitrogen then gets assimilated into organic compounds within the plant. The process involves the conversion of nitrate to nitrite (NO2-) before changing it further into ammonium ions, followed by its incorporation into amino acids via the glutamine synthetase-glutamate synthase (GS-GOGAT) pathway. These amino acids are later on used for synthesizing proteins, nucleotides and other nitrogenous compounds important for growth and development.
Nitrogen Use Efficiency (NUE) is the ratio between grain yield and applied N (kg grain/kg N). High NUE indicates an efficient use of N, implying that more productivity can be achieved at lower environmental costs. For instance, temperate cereal crops range between 30 and 50 kg grain/kg N applied in terms of their NUE values.
- Nitrogen assimilation rate: Refers to the conversion efficiency of absorbed nitrogen into biomass, generally measured in mg of N converted per gram of plant tissue per hour.
- Glutamine synthetase activity: A key enzyme measured in units (U) per mg of protein, where 1 unit corresponds to the amount of enzyme forming 1 µmol of glutamine per minute.
- Nitrate reductase activity: Indicates the efficiency of nitrate to nitrite reduction, typically given in µmol of NO2- produced per gram of fresh weight per hour.
- Nitrogen Use Efficiency (NUE): Measured in kg of grain per kg of nitrogen applied, with higher values suggesting more efficient nitrogen usage.
By understanding and optimizing these parameters, agricultural practices can be refined to enhance nitrogen uptake and utilization, ultimately leading to more sustainable and productive farming systems.
What Are the Different Forms of Nitrogen Available to Plants?
Nitrate (NO3-) and ammonium (NH4+) are the major forms of nitrogen utilized by plants. Nitrate is the most common form of nitrogen taken up by plants, particularly in well-aerated soils. Uptake and assimilation involve the enzyme nitrate reductase that catalyzes reduction of nitrate to nitrite. This step is technical and significant, typically measured in µmol of NO2- produced per gram fresh weight per hour. However, Ammonium which is less abundant in soil plays a major role as a nitrogen source for direct assimilation into amino acids through glutamine synthetase.
Quantification of glutamine synthetase activity is commonly expressed in units (U) per mg protein thereby indicating its ability to synthesize 1 µmol glutamine within 1 minute.
Besides nitrate and ammonium, some organic forms of nitrogen, such as amino acids and peptides, can be used by plants, especially in nutrient-depleted soils. In this case, the organic forms are incorporated directly into plant metabolism without being subjected to nitrate reductase or/and glutamine synthetase activities.
Agricultural systems should recognize and manage these different forms of nitrogen with their respective utilization efficiencies to optimize nitrogen application strategies for increased crop yields while minimizing environmental impacts.
How Does Nitrogen Deficiency Affect Plant Growth?
Plant growth will be significantly retarded by lack of nitrogen and various symptoms will signal about disrupted cellular activities. Chlorosis is one of the leading signs which means leaves become yellow beginning from those that are positioned at the bottom, then it spreads upwards to other parts. Thus, nitrogen is important in chlorophyll which is the main molecule responsible for photosynthesis. It causes a reduction in chlorophyll content which in turn lowers photosynthetic rate leading to limited light energy conversion into chemical energy.
Also, plants lacking nitrogen exhibit stunted grown and have less biomass production. This occurs as a result of reduced amino acid synthesis since Nitrogen is a key element in amino acids, the building blocks of proteins. The levels of proteins also decline resulting in deficiencies in essential enzymes and structural components necessary for normal development.
Again, there are lower concentrations of cytokinins and phytohormones that promote cell division and shoot formation due to nitrogen deficiency. Consequently, hormonal imbalances adversely affect root growth, resulting in poor root development and decreased shoot formation, further reducing nutrient uptake and water availability. The physiological parameters affected by nitrogen deficiency include:
- Chlorophyll Content: Measured in SPAD units or µg/cm².
- Photosynthetic Rate: Measured in µmol CO2/m²/s.
- Amino Acid Concentration: Measured in nmol/mg fresh weight.
- Biomass Accumulation: Recorded as g/plant or kg/ha.
By monitoring these technical parameters, farmers and agronomists can diagnose nitrogen deficiency accurately and implement corrective measures to restore optimal plant health and productivity.
How Does the Nitrogen Cycle Work?
The nitrogen cycle is a complex biogeochemical process with many key steps that help nitrogen and its compounds to transfer among the atmosphere, biosphere, and geosphere. Nitrogen fixation initiates the process, where atmospheric nitrogen (N₂) is converted into ammonia (NH₃) by nitrogen-fixing bacteria or through industrial processes. Then nitrification occurs in which nitrites (NO₂⁻) and nitrates (NO₃⁻) are formed from the ammonia by soil nitrifying bacteria. These nitrates are absorbed by plants from soil via root systems to manufacture important molecules such as proteins and amino acids.
The cycle continues after plants assimilate nitrogen compounds through herbivores and carnivores who include this nutrient into their biological systems. Ammonia that has been released back into the ecosystem is then decomposed again by other organisms, including fungi and bacteria, when animals or plants die.
Lastly, denitrifying bacteria convert nitrates back into atmospheric nitrogen (N₂) in a process called denitrification, thus completing the cycle. All these activities aim to balance the nitrogen supply in ecosystems so that it remains available for ongoing biological production and growth.
What is Nitrogen Fixation?
Nitrogen fixation is an essential process whereby gaseous N₂ in the atmosphere is converted into NH₃, which plants can use. It may happen abiotically, as seen within industries like the Haber-Bosch method, or biotically through symbiotic bacteria, for instance, Rhizobium, which forms associations with leguminous plants primarily. These bacteria carry an enzyme named nitrogenase, which transforms non-reactive atmospheric nitrogen to bioavailable ammonia for further conversion to nitrites and nitrates using soil bacterium enzymes. Nitrogen fixation must occur because soils need to be replenished with it, so that plant can grow well while maintaining ecosystem productivity.
The Role of Organic Nitrogen and Mineral Nitrogen in the Cycle
Nitrogen cycle involves organic nitrogen, which is found mainly in living organisms or decomposing matter. Decomposition of plant and animal remains is a key way through which it enters into the soil. Consequently, soil microorganisms mineralize this organic nitrogen to ammonia (NH₄⁺) and nitrates (NO₃⁻), which are useful forms for plants.
Mineral nitrogen refers to nitrogen in its nonliving forms, such as ammonium and nitrates. These are very significant since they form the major uptake by plant roots from the soil for nutrition purposes. Further amalgamation of hydrogen with oxygen may create some nitrifying bacteria, which converts NH4+ into nitrite NO2—and then finally nitrate NO3-. Most plants prefer nitrates to other sources of nitrogen.
In combination, these two forms, organic and mineral nitrogen, make up an important nutrient cycle that provides continuous nitrogen availability for plant growth throughout their life cycles and supports ecosystem productivity as a whole.
What Are the Key Processes in the Nitrogen Cycle?
The nitrogen cycle comprises of several important steps: Nitrogen fixation, nitrification, assimilation, ammonification and denitrification. Nitrogen fixation is the process in which atmospheric nitrogen (N₂) changes to biologically useful forms such as ammonia (NH₃). Specifically, this is done by some bacteria and archaea that are symbiotically related to plants. Followed by this is Nitrification, whereby ammonia is first oxidized into nitrites (NO₂⁻) and then nitrates (NO₃⁻) by nitrifying bacteria.
Assimilation involves the uptake of nitrates and ammoniums by plants to synthesize organic molecules like amino acids and proteins. Ammonification, also known as mineralization, happens when dead organisms’ organic nitrogen, together with other waste products, are converted back to ammonia and ammonium ions through the decomposition of microorganisms. Finally, denitrification occurs where nitrates are reduced into gaseous nitrogen (N₂) or nitrous oxide (N₂O) by denitrifiers returning it back into the atmosphere, thus completing the nitrogen cycle. These processes help keep equilibrium in ecosystems’ nitrogen, making it available for biological use but still ensuring its continuous movement across the environment.
Why Do Plants Need Nitrogen Fertilizer?

Plants need nitrogen to survive because it is an essential constituent of chlorophyll, proteins and nucleic acids that are key for photosynthesis, protein synthesis and other processes that govern plant development and growth. Often, the natural level of nitrogen in soils is too low to allow plants to reach their full potential; hence, farmers have to fertilize with nitrogen-based products. These fertilizers ensure enough nitrogen is available for plant uptake, consequently leading to strong growth and high crop yields and quality. This is especially important in agricultural systems where soil N may be depleted by practices such as over-farming, thus making the addition of more fertilizer a necessity for food production and continuous sustainability.
How Does Nitrogen Fertilizer Enhance Plant Growth?
Nitrogen fertilizers boost plant growth through different pathways but mainly by acting as an instant source of available nitrogen which plants can take up. It aids in forming amino acids, proteins and nucleic acid, which are vital biochemical components. By increasing the soil content of nitrogen when applied on it, these types of manure facilitate its easy uptake by plants. In this regard, the rate at which chlorophyll is produced (this being the pigment responsible for photosynthesis) increases dramatically upon adding nitrate fertilizers into the soil. An increase in chlorophyll synthesis enhances light energy absorption capacity by plants; hence more efficient photosynthesis resulting in robust development.
Technical parameters supporting these benefits include:
- Nitrogen Uptake Efficiency (NUE): A higher NUE means that plants absorb a greater proportion of the nitrogen applied as fertilizer, directly correlating to enhanced growth rates.
- Chlorophyll Concentration: Studies show that plants with higher nitrogen availability exhibit increased chlorophyll concentration, which boosts photosynthetic rates by up to 50%.
- Yield Improvement: Proper nitrogen fertilization can increase crop yield by 20-50%, depending on the plant species and growing conditions.
- Root Development: Nitrogen fertilizers promote root growth and development, enhancing the plant’s ability to absorb water and other nutrients, further supporting overall plant health and productivity.
These detailed mechanisms and parameters underscore the critical role of nitrogen fertilizers in optimizing plant growth and ensuring high agricultural productivity.
What Are the Types of Nitrogen Fertilizers?
- Urea (CO(NH₂)₂):
-
- Description: Urea is the most commonly used nitrogen fertilizer globally, accounting for over 50% of world nitrogen fertilizer consumption.
- Nitrogen Content: Approximately 46% nitrogen by weight.
- Application Method: Urea is typically applied to soil as granules or in a liquid form. It can also be sprayed directly onto foliage.
- Efficiency: High nitrogen release efficiency, but subject to volatilization losses unless incorporated into the soil or applied with inhibitors.
- Ammonium Nitrate (NH₄NO₃):
-
- Description: A highly soluble and versatile nitrogen fertilizer, ammonium nitrate is often used in regions requiring rapid nitrogen uptake.
- Nitrogen Content: Contains 34% nitrogen by weight.
- Application Method: Applied in granule form, either broadcast on the soil surface or localized in bands.
- Efficiency: It quickly releases nitrogen, half in the fast-acting nitrate form and half in the slower ammonium form.
- Ammonium Sulfate ((NH₄)₂SO₄):
-
- Description: Often used when soil pH needs to be lowered, as it has an acidifying effect.
- Nitrogen Content: Contains approximately 21% nitrogen by weight.
- Application Method: Typically applied as granules, they can be dissolved in water for fertigation.
- Efficiency: Offers slower nitrogen release compared to urea and ammonium nitrate, benefiting long-term crop growth.
- Calcium Ammonium Nitrate (CAN):
-
- Description: A safer alternative to ammonium nitrate, especially favored in regions with strict regulations on nitrate fertilizers.
- Nitrogen Content: Contains about 27% nitrogen by weight.
- Application Method: Generally applied in granule form or mixed with other nutrients in compound fertilizers.
- Efficiency: Provides balanced nitrogen availability, enhancing nutrient uptake and reducing leaching risks.
- Anhydrous Ammonia (NH₃):
-
- Description: The most concentrated form of commercial nitrogen fertilizer.
- Nitrogen Content: Contains 82% nitrogen by weight.
- Application Method: Injected into the soil to prevent gaseous losses.
- Efficiency: Excellent nitrogen efficiency when properly applied, offering both immediate and prolonged availability.
These various forms of nitrogenous fertilizers have different advantages and properties, which make their targeted use to optimize plant nutrition for improved crop yields possible. A detailed understanding of each permits accurate and efficient fertilizer management in diverse agricultural contexts.
How Much Nitrogen Is Necessary For Optimal Crop Growth?
The exact nitrogen requirement for optimum crop growth varies greatly among different crops, types of soil, and environmental conditions. The following are some general recommendations and technical parameters according to the findings from leading agricultural websites:
- Corn: Typically requires between 1.2 to 1.5 pounds of nitrogen per bushel of expected yield. For example, a target yield of 200 bushels per acre would necessitate approximately 240 to 300 pounds of nitrogen per acre.
- Wheat: Optimal nitrogen rates range from 1.5 to 2.0 pounds per bushel of expected yield. A target of 80 bushels per acre would require about 120 to 160 pounds of nitrogen per acre.
- Soybeans: Though soybeans fix atmospheric nitrogen through symbiotic relationships with soil bacteria, supplementing with 20 to 30 pounds of nitrogen per acre can sometimes enhance yields, particularly in poor soils or when grown as a second crop.
- Rice: Generally requires about 100 to 150 pounds of nitrogen per acre, depending on the variety and environmental conditions.
Soil organic matter content, crop residues from previous season, and irrigation practices are some of the nitrogen demand determinants. For example, soils with a high content of organic matter require less added nitrogen due to the release of nutrients that is inherent in decomposition. Moreover, efficient irrigation management can reduce leaching and volatilization losses of nitrogen hence leading to an optimized NUE by nitrogen. In order to provide precise rates for applying N through using soil tests and crop growth models, one should follow analysts’ advice which will ensure both economic and environmental sustainability.
How Is Soil Nitrogen Managed for Plant Growth?

Soil nitrogen management for plant growth is a combination of practices that aim to maximize the availability of nitrogen while minimizing environmental damage. These include soil tests, crop rotations and the use of nitrogen fixing cover crops. Soil testing establishes background levels of nitrogen and guides application rates so as not to over- or under-fertilize crops. Crop rotation, especially with legumes that fix atmospheric nitrogen can enhance natural soil fertility through increased soil N content. When they decompose, such cover crops like clover or alfalfa can also add organic nitrogen to the soil. Also, controlled-release fertilizers and precision agriculture techniques such as variable rate technology (VRT) ensure that the right amount of nitrogen fertilizer is applied at the right time for optimum crop uptake. As a result of these initiatives, farmers will be able to have improved NUE and sustainable agricultural practices.
Why Is Nitrogen Important in Soil?
Nitrogen is an indispensable component necessary for plant growth and development. It is one of the important macronutrients, forming part and parcel of amino acids, proteins, nucleic acids, and chlorophyll, which are vital for cellular activities, including photosynthesis. Lack of adequate supply often causes poor performance involving leaf yellowing due to chlorosis leading to stunted growth.
From a technical viewpoint, there are several forms in which it takes in soil before undergoing complex transformations entailing mineralization nitrification as well as denitrification respectively. For instance, major factors affecting all these processes may include pH value in soils ranging from 4-8; temperature fluctuation; water contents, hence moisture availability; presence/absence of organic matter within it, amongst others, dictates how the process moves along its cycle. The optimum pH range in soils considered good for nitrogen release varies from 6-8.Temperatures affect microbial activity where nitrification peaks between 25oC-35oC temperatures.Adequate moisture should always be ensured during microbial processes but excess of water may bring about nitrogen losses through leaching and denitrification.
In order to achieve accurate monitoring and management of nitrogen the soil must be subjected to constant testing and precision agriculture instruments. These, in conjunction with the appropriate application of controlled-release fertilizers, can facilitate efficient use of nitrogen while minimizing environmental impacts, thereby enhancing sustainable agricultural systems.
How Can Farmers Enhance Nitrogen Availability in Soil?
Several strategies can be employed by farmers to enhance nitrogen availability in soils, ensuring efficient nutrient use and sustainable agricultural practices.
- Soil Testing and Monitoring: Performing regular soil tests helps determine the current nitrogen levels and other essential parameters such as pH, organic matter content, and microbial activity. Technologies like remote sensing and precision agriculture tools provide detailed spatial and temporal data, allowing for tailored nutrient management plans.
- Use of Leguminous Cover Crops: Integrating leguminous cover crops, such as clover, alfalfa, and beans, can naturally enhance soil nitrogen through biological nitrogen fixation. These plants harbor symbiotic bacteria (Rhizobium spp.) in their root nodules, which convert atmospheric nitrogen (N₂) into ammonia (NH₃), a form readily available to plants.
- Application of Controlled-Release Fertilizers: Controlled-release fertilizers (CRFs) and urease inhibitors ensure a steady nitrogen supply by preventing rapid leaching and volatilization losses. The nitrogen release rate from these fertilizers depends on factors such as soil temperature and moisture content. Typical parameters include the nitrogen release profile (e.g., 30% over 3 months, 70% over 6 months).
- Maintaining Proper Soil pH: Ensuring the soil pH is within the optimal range of 6 to 8 improves nitrogen availability. Lime (calcium carbonate) can be applied to acidic soils to raise pH, while sulfur compounds can lower pH in alkaline soils, thus promoting efficient nitrogen uptake.
- Minimizing Soil Erosion and Leaching: Implementing practices like contour farming, strip cropping, and maintaining vegetation cover helps reduce soil erosion and surface runoff, conserving soil nitrogen. Additionally, proper irrigation management prevents excess water which could lead to nitrogen leaching into deeper soil layers or groundwater.
Adopting these strategies can help farmers improve the efficiency of nitrogen use. They will be able to optimize their crop yields and minimize the environmental implications of using nitrogenous fertilizers.
What are some of the Best Practices in Nitrogen Management?
In order to effectively manage nitrogen, several best practices must be incorporated with regard to specific crop requirements. One significant method is implementing precision agriculture techniques where one uses sophisticated methodologies like GPS, remote sensing among others in order to provide accurate nitrogen fertilizer application. This makes sure that different growth stages’ crops acquire precisely required amounts of nitrogen thereby eliminating wastages and environmental pollution.
Another important practice is using cover crops, which fix atmospheric nitrogen and enhance soil structure. Specifically, leguminous cover crops can increase the availability of N through decomposition, providing an organic source of this nutrient for subsequent plants. Nitrogen-fixing plants such as legumes, when put into crop rotation, also act as good examples for maintaining soil fertility and breaking pest cycles.
Finally, irrigation management must be optimized. There may be leaching of excess water if farmers over-irrigate while under irrigation, causing stress to the plants due to lack of enough water or moisture. Nitrogen utilization would greatly benefit from systems like trickle/ drip irrigation with scheduled watering based on soil moisture sensors among others. In this way, farmers can achieve sustainable and productive nitrogen management through adoption of these sophisticated practices.
What Are the Benefits of Nitrogen Use Efficiency?

The importance of enhancing nitrogen use efficiency (NUE) is many. The most significant one is that it enhances agriculture by ensuring that plants get enough nitrogen for maximum growth leading to increased yields and improved crop quality. This in turn decreases the amount of nitrogen being washed out of the soil due to leaching, volatilization, or runoff thus reducing water pollution and greenhouse gas emissions. Financially, effective use of nitrogen results in reduced expenditures on purchasing and applying fertilizers thus enabling farmers to make more profits. Furthermore, proper management of N not only sustains soil fertility but also support microbial activity which help maintain nutrient balance in soils for long term agricultural productivity and health.
How Can Nitrogen Use Efficiency Be Improved?
- Precision agriculture:
- Soil Testing and Leaf Analysis: Regular soil tests and leaf tests can be used to provide accurate data on nitrogen levels which can inform selective fertilization. This will reduce misuse of fertilizers that may result in over-application and ensure that plants receive only required amount of nitrogen.
- Variable Rate Technology (VRT) relies on GPS guidance and sensors to change the rate of fertilizer application across a field. By doing so, areas with low nitrogen demand are given minimal amounts, while areas with high nitrogen requirement are adequately supplied.
- Enhanced Efficiency Fertilizers (EEF):
- Slow Release Fertilizers: These kinds of fertilizers release nitrogen slowly as the crop grows. Consequently, they reduce nitrate leaching and volatilization.
- Nitrification Inhibitors and Urease Inhibitors delay the process of nitrogen transformation in soil, thus prolonging its availability to plants.
- Optimized Irrigation Practices:
- Drip Irrigation Systems: They supply water directly into the root zone thereby minimizing water wastage and loss nutrients. Combining this with real-time soil moisture sensors helps them maintain optimum soil moisture content.
- Scheduling Based on Climate Data: Therefore, by utilizing weather predictions alongside evapotranspiration information, farmers can plan their irrigation timetables to prevent excess water from draining off and carrying away nitrates.
By incorporating these approaches together with robust data collection and analysis, farmers will be able to significantly improve NUE hence sustainable farming practices that make more money.
What Are the Environmental Impacts of Efficient Nitrogen Use?
Some positive changes that come with efficient nitrogen use are highlighted here. First, it helps in reducing the amount of nitrogen leachates into the ground water aquifers which affects drinking water sources and aquatic habitats adversely. Besides, better NUE reduces nitrous oxide emission, a powerful greenhouse gas contributing to climate change. Moreover, it curtails nitrogen discharge into rivers and lakes causing toxic algal blooms and eutrophication leading to dead zones where no marine life can live. Soil fertility is also enhanced alongside biodiversity in agro-ecosystems as farmers optimize their fertilization schedules.
What Is the Connection Between Plants and Animals in the Nitrogen Cycle?
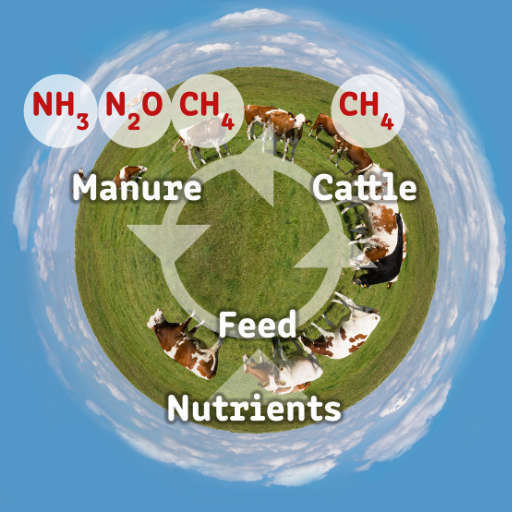
Nitrogen cycle involves plants and animals as its crucial components; they contribute to conversion of nitrogen within the ecosystem between different forms. Nitrogen in the soil is mainly absorbed by plants in form of nitrates and ammonium ions, which are important for formation of amino acids, proteins and other important organic compounds. Herbivorous animals and omnivores feed on these plants thereby transferring plant-based nitrogen into their own body tissues. Bacteria and fungi decompose organic nitrogen during elimination of animal wastes or death of plants or animals, thus releasing it back to the soil as ammonium. On the other side, fixed ammonia then gets converted to nitrate by nitrifying bacteria closing this circle that again makes nitrogen available for uptake by plants. Moreover, leguminous plants have root nodules that harbor bacteria capable of fixing atmospheric nitrogen to useful forms usable by the plant hence enhancing soil with vital nutrients further
What Is the Contribution of Plants and Animals to Nitrogen Fixation?
Plants’ primary role in the nitrogen cycle is through nitrogen uptake and assimilation. Therefore, they absorb it from soils mainly as nitrates and ammonium ions, which are vital for synthesis of amino acids and proteins. Nitrogen-fixing plants like legumes also have symbiotic bacteria in their root nodules that convert atmospheric nitrogen into a form that can be used by the plant. On the other hand, animals take up this nitrogen by feeding on these plants. When such animals defecate or die, there are certain decomposers like bacteria and fungi that break down organic matters thereby releasing nitrogen back into soil as ammonium.
Why do Plants & Animals Fix Nitrogen?
One of the most significant processes in the course of the nitrogen cycle is known as nitrogen fixation; each type plays its own specific part in this process with regards to both animals and plants involved. In particular, some plants, particularly legumes, enter into a symbiosis with rhizobia bacteria which colonize root nodules. In addition to enhancing soil fertility for other species this mutualistic relationship between host plant and Rhizobium sp helps supply an essential nutrient for the former’s growth.
Animals contribute indirectly towards nitrogen fixation through support of ecosystems where there are such plants growing. For instance, their activities like grazing can help control plant numbers while at the same time promoting growth of species which fix nitrogen. Besides that when animals excrete waste materials or decompose; they provide organic matter that can support different forms of soil microorganisms including those that fix nitrate thereby maintaining a healthy amount within an ecosystem.
Frequently Asked Questions (FAQs)
Q: Why is nitrogen important to plants?
A: Nitrogen is an essential nutrient for plants, playing a critical role in their vegetative growth. It is a component of vital compounds such as amino acids, proteins, and chlorophyll. Without adequate nitrogen, plant growth is significantly stunted.
Q: How do plants obtain nitrogen from the soil?
A: Plants obtain nitrogen from the soil primarily in the form of nitrate or ammonium ions. These forms of nitrogen are easily absorbed through the plant roots and used in various metabolic processes.
Q: What happens if there are low levels of nitrogen in the soil?
A: Low levels of nitrogen in the soil can lead to poor plant growth, yellowing leaves, and reduced crop yields. Such deficiencies highlight the importance of maintaining an adequate nitrogen supply for healthy plant development.
Q: Can plants grow using atmospheric nitrogen?
A: While atmospheric nitrogen makes up about 78% of the air, most plants cannot use it directly. Certain bacteria in the soil convert atmospheric nitrogen into forms that plants can absorb—a process known as nitrogen fixation.
Q: What role does nitrogen fertilization play in agriculture?
A: Nitrogen fertilization is crucial in agriculture as it provides a readily available source of nitrogen for crop plants, thus enhancing their growth and productivity. Proper nitrogen application ensures higher crop yields and better quality produce.
Q: What are the risks of excessive nitrogen fertilization?
A: Excessive nitrogen fertilization can lead to nitrogen losses through leaching and runoff, causing environmental pollution. It may also result in poor plant health due to imbalanced nutrient uptake.
Q: How can farmers increase nitrogen levels in the soil naturally?
A: Farmers can increase nitrogen levels in the soil naturally through practices such as crop rotation, planting nitrogen-fixing crops like legumes, and using organic matter like compost and manure to enhance the availability of nitrogen.
Q: Why is plant nitrogen metabolism significant?
A: Plant nitrogen metabolism is significant because it involves the transformation and utilization of nitrogen for the synthesis of amino acids, proteins, and other essential compounds. This process is vital for plant development and overall health.
Q: How does nitrogen impact shoot growth in plants?
A: Nitrogen impacts shoot growth by promoting the elongation and proliferation of plant cells. Adequate nitrogen ensures robust shoot growth, resulting in more extensive foliage, which is crucial for photosynthesis and energy production.
Q: What happens to higher plants with inadequate nitrogen supply?
A: Higher plants with an inadequate nitrogen supply exhibit stunted growth, reduced leaf size, and yellowing foliage (chlorosis). This deficiency severely affects the plant’s ability to perform photosynthesis and produce sufficient energy for growth.






