The interaction of nitrogen within an ecosystem stems from the nitrogen cycle, a biogeochemical activity essential for regulating nitrogen levels in the environment. In contemporary agriculture, fertilizers are important for improving soil and crop productivity. However, applying fertilizers alters the nitrogen cycle in more profound ways, the most notable of which is the disruption of natural processes.
Thus, this article analyzes the relationship between using fertilizers and the nitrogen cycle and its benefits, but with special consideration of the negative impacts. The main subjects of concern are the mechanisms whereby fertilizers N transformations in the soil, possibilities of N risks through leaching or N emissions, and even broader relationships to ecosystems and climate change. With this analysis, this article purports to provide information on the impact of fertilizers on the balance of the nitrogen cycle and how these negative impacts can be reduced.
What is the Nitrogen Cycle, and How Does it Work?
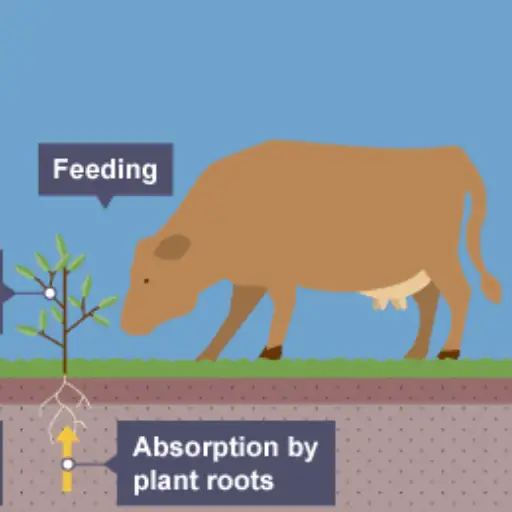
The nitrogen cycle describes the transformations and movements of nitrogen within the atmosphere, biosphere, and geosphere. It is a biogeochemical process whereby nitrogen, a vital element for all living entities, is interchanged between different chemical forms—nitrogen gas (N₂), ammonium (NH₄⁺), nitrites (NO₂⁻), and nitrate (NO₃⁻). It mainly involves five processes: nitrogen fixation, ammonification, nitrification, assimilation, and denitrification.
Atmospheric nitrogen gas can be transformed into ammonia by specialized bacteria or an industrial process known as nitrogen fixation. In this form, ammonia can easily be used by living organisms. The next stage is the conversion of ammonia into nitrites and, later on, to silicate, which plants can utilize; this stage is called nitrification. This form of nitrogen is converted to organic forms like amino acids and proteins during incorporation, also known as assimilation, in plants and other organisms. Ammonification is executing the reverse of the nitrogen cycle by the decomposition of organic matter to obtain ammonia. Last in the cycle is denitrification, which returns nitrogen to the atmosphere in the form of nitrogen gas through anaerobic bacteria. Anthropogenic activities such as using fertilizers lead to an ecological imbalance of nitrogen, creating ecological and environmental challenges.
Understanding the Basic Steps of the Nitrogen Cycle
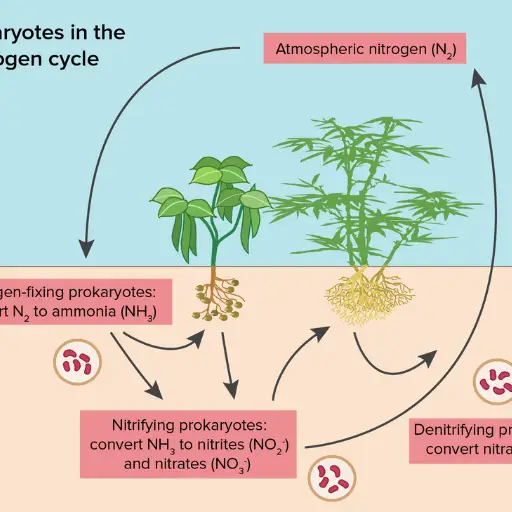
The nitrogen cycle comprises several processes that allow nitrogen to traverse through varying ecosystems. First, nitrogen fixation makes nitrogen in the air usable through different forms of microbial life or irrigation. Ammonia is then transformed into nitrites and subsequently into plant-usable nitrates during Nitrification. Plants nitro and animals incorporate it into organic molecules during Assimilation. Nitrogen is recycled as ammonium while decomposing organic matter, and ammonia gas is released into the atmosphere during Denitrification. These processes are interrupted by human actions like the overuse of fertilizers, leading to polluted waters and increased greenhouse gases in the atmosphere.
The Role of Nitrogen Fixation in the Nitrogen Cycle
Nitrogen fixation refers to the steps that convert nitrogen gas into ammonia in the atmosphere, which living organisms can use. Nitrogen gas is added to the nitrogen cycle and can be obtained from biological and physical sources. Biological nitrogen fixation happens with the help of bacteria like Azotobacter and Rhizobium, which are free-living and symbiotic, respectively. These microbes have the nitrogenase enzyme, which converts nitrogen gas into ammonia. This happens in anaerobic conditions and requires about 16 ATP molecules for each nitrogen molecule.
In simpler terms, Ammonia can also be produced without biological tools. For instance, lightning can split nitrogen gas into nitrogen oxides, which are easily absorbed into soil. These mechanisms can aid in the formation of ammonia. The Bosch process produces ammonia from nitrogen and hydrogen gas at extremely high temperatures (400 – 500 Celsius) and under extreme pressures (150-200 atm) with an iron catalyst. This process creates 150 million metric tons of ammonia annually, which maintains the needs of agriculture.
Both soil fertility and plant productivity are directly influenced by nitrogen fixation. Its imbalance caused by humans, particularly from over-application of synthetic fertilizers, worsens the problem of nitrogen deposition in the atmosphere, which creates further environmental issues like eutrophication, loss of biodiversity, climate change, and even more significant emission of nitrous oxide (N₂O). It is also necessary to manage the application of fertilizers and encourage biological fixation to ensure that the nitrogen cycle’s balance is never disrupted.
How Nitrogen Moves Through Different Ecosystems
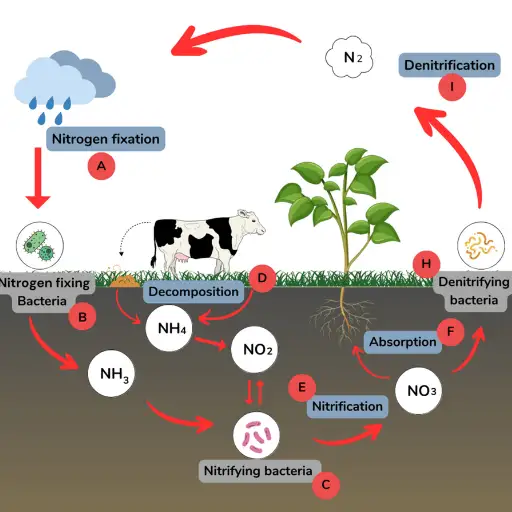
The transfer of nitrogen to a variety of ecosystems relies heavily on the nitrogen cycle and its key components: nitrogen fixation, nitrification, assimilation, ammonification, and denitrification. All processes maintain life by efficiently managing the nitrogen distribution in the soil, water, atmosphere, and other living organisms.
- Fixation of Nitrogen
Nitrogen Fixation starts when 78% of the earth’s atmosphere turns into bioavailable forms like ammonia and nitrates. Biological fixation is where it all begins, and it is executed by friendly bacteria like Rhizobium or Bradyrhizobium, which produces a cumulative 100 million metric tons annually. It is important to note that not all nitrogen gets consumed in biological fixation. A good portion of it gets converted during lightning strikes. Nitrogen turns into nitrogen oxides that mix with rainwater, providing soil nutrients.
- Nitrification
The specialized soil bacteria take it from here. Through a two-step aerobic process, the bacteria converts ammonia into nitrite while simultaneously changing it into nitrite and knocking it down into nitrate. These conversions are especially important for plant growth and vital for the soil-nurturing bacteria. The ideal conditions for Nitrogen Fixation are 20 to 30 degrees in temperature and a pH range of 6 to 8.
- Assimilation
Nitrates and ammonium are transmuted into nitrogen-based proteins, nucleic acids, and core biomolecules, which are absorbed through the plant’s roots. Herbivores and other carnivorous species absorb these nitrogen compounds by consuming the herbivores.
- Ammonification
Bacteria and fungi decompose organic nitrogen compounds from dead life forms into ammonium, which is then transformed into ammonium. This process is key in enhancing soil nitrogen concentration, which is important for plant and microbial activity.
- Denitrification
The transformation of nitrogen dioxide to nitrogen is outlined as denitrification and occurs in biological ecosystems where oxygen concentrations are low, such as in some soils or water bodies. [[Pseudomonas]] and [[Paracoccus]] are examples of denitrifying bateria. This enables the gaseous nitrogen concentration to be reduced in biological systems, but it also increases the atmospheric nitrogen concentration while contributing to nitrous oxide. This process is less active in agriculturally-oriented places, without much organic matter.
These interconnected processes exchange nitrogen between terrestrial, aquatic, and atmospheric ecosystems. Nonetheless, the unnatural nitrogen flows that farmers have instigated and the burning of fossil fuels underline the importance of sustainable approaches to lessen the impact on the ecosystems.
How Does the use of fertilizer affect the nitrogen cycle?
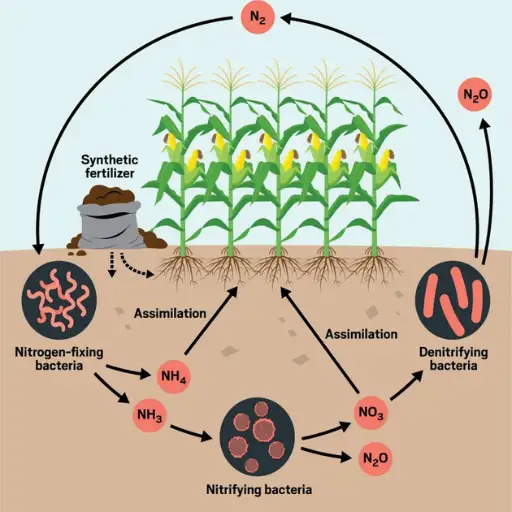
Fertilizer application alters the nitrogen cycle by adding large quantities of reactive nitrogen in the form of ammonium (NH₄⁺) and nitrate (NO₃⁻). Although this enhances soil fertility and increases crop production, a significant fraction of the nitrogen frequently leaches into groundwater or runs off into water bodies, resulting in eutrophication and hypoxia. In addition, the surplus nitrogen compounds increase the rate of denitrification, which further increases the emission of nitrous oxide (N₂O) – one of the most important greenhouse gases. Eventually, such disturbances result in poor soil conditions and water pollution and further aggravate climate change, demonstrating the importance of judicious use of fertilizers and effective nutrient management.
The Contribution of Nitrogen from Fertilizers
In my opinion, fertilizers are a significant source of environmental reactive nitrogen. When used excessively or inefficiently, fertilizers add nitrogen compounds such as ammonium and nitrate into ecosystems. This dramatically increases agricultural productivity but comes with adverse side effects. For example, nitrogen that rushes off can promote eutrophication in aquatic ecosystems, resulting in a hypoxic zone detrimental to most marine creatures. In addition, a portion of this nitrogen is released into the atmosphere as nitrous oxide, a potent greenhouse gas. To combat these problems, I believe it is essential to take more care when applying fertilizers and includes sustainable practices such as using slow-release fertilizers and crop rotations to enhance productivity and conserve the environment at the same time.
Excessive Use of Fertilizers and Its Effects
Fertilizers are essential for modern agriculture, and one side effect of this is the massive degradation of farmland. From what I have read, the newest studies show that overapplication contributes to soil degradation, water contamination, and greenhouse gas emissions. Over a short period, eutrophication of water bodies due to agricultural runoff caused by massive algal blooms is bound to happen. Furthermore, the algal blooms decrease the overall biodiversity of the body of water. Not to mention, the release of nitrous oxide is a key contributing factor to increased climate change. I believe that by adopting precision farming, using organic soil amendments, and improving soil moisture and nutrient content monitoring, I can resolve these issues and practice sustainable agriculture.
Impact of Fertilizers on Nitrogen Levels in the Soil
The application of fertilizers does alter the nitrogen concentration in soil positively and negatively simultaneously. Using fertilizers with high nitrogen content, such as ammonium nitrate or urea, increases soil fertility by replacing blocked nitrogen. However, with the lack of management, fertilizer use can damage the environment with nitrogen surplus. Other important details are:
- NITROGEN USE EFFICIENCY (NUE): In conventional farming, it is always best to have more than 30% to 50% of the crop’s efficiency. This is a best practice to maximize the crop’s ability to use nitrogen.
- APPLICATION RATE: The proper application of nitrogen as a fertilizer differs among different crops. For example, cereals need about 100-150 kg/ha during planting.
- LEACHING POTENTIAL: The possibility of nitrogen leaching rises when soil nitrate content is above 50 mg/L, where the soil sand bed is easily leached.
- VOLATILIZATION LOSSES: In warm and moist environments, the losses double. Luckily, if urea-based fertilizers are incorporated well into the soil, ammonia gas will not be released.
On the other hand, the Thai approach includes using controlled-release or slowly soluble nitrogen fertilizers and scheduling split applications specific to the crops’ needs. He also recommends frequent checking of soil nitrogen concentrations to set a proper baseline for fertilizing management techniques.
What Role Do Nitrogen-Fixing Bacteria Play?
The bacteria in legumes, such as Rhizobium and free-living bacteria Azotobacter, perform essential functions. They are considered nitrogen-fixing bacteria, which means they transform atmospheric nitrogen (N2) into a more readily available substance, like ammonia (NH3), for organisms to use. These microorganisms use unique enzymes like nitrogenase to cleave the strong bonds in nitrogen gas. This process increases natural nitrogen availability and reduces the need for synthetic fertilizers. It also enhances soil fertility. In addition, their symbiotic relationship with plants helps advance agriculture because it increases crop production and lessens the negative impact on the ecosystem.
How Nitrogen-Fixing Bacteria Affect the Nitrogen Cycle
The conversion of atmospheric nitrogen (N₂) to biologically usable forms requires nitrogen-fixing bacteria, which function in and around ammonium (NH₃). These bacteria play an essential role in the nitrogen cyclic process by mediating the unused nitrogen in the atmosphere via a similar process to biological nitrogen fixation. The entire transformation process starts when nitrogen-fixing bacteria structure with the nitrogenase enzyme system. The whole process of anaerobic severs uses energy, requiring significant energy input. On average, a nitrogen-fixing bacterium spends roughly N₂ ATP molecules to explode, which makes this an essential but active sea life process.
Biological nitrogen fixation systems have specific technical parameters like:
1. Substrate: Nitrogen (N₂) in the form of gas.
2. Enzyme: Nitrogenase complex that contains Molybdenum – Iron or Vanadium – Iron cofactors.
3. Required Conditions: Anaerobic or microaerophilic conditions and accessible energy in AT.
4. End Product: Ammonia (NH₃), which pens may further assimilate plants into other nitrogen compounds within the soil.
5. Energy Expenditure: Ammonia fed the soil restrains these inoculums and crops, letting them burst afterward, with approximately 8 electrons.
The activity of B fixers is the essential input in the nitrogen cycle. It changes the oxygen-dependent nitrogen availability, minimizes the dependency on chemical nitrogen fertilizers, and prevents nitrogen depletion in agriculture.
The Relationship Between Nitrogen Fixation and Fertilizer Use
Using fertilizers makes it very easy to enlist the assistance of nitrogen-fixing bacteria for crops, as these crops provide the necessary nitrogen-fixing capacity. Some symbiotic bacteria, like Rhizobium, and free-living organisms, like Azotobacter, all help do biological nitrogen fixation. These organisms take nitrogen directly from the atmosphere and transform it into ammonia, which plants can use, eliminating the need for synthetic nitrogen fertilizers. This biologically-acquired nitrogen can replace one or more chemical fertilizers. This results in reduced costs and fewer ecological threats, such as the eutrophication of water bodies due to excessive nitrogen. Selectively integrating nitrogen-fixing crops within cropping systems can improve soil health while increasing sustainability in farming. Therefore, achieving biological nitrogen fixation is an intelligent method to ensure ecological care while meeting the requirements for productivity.
How Does Fertilizer Use Affect Aquatic Ecosystems?
Using fertilizer threatens aquatic life due to nutrient runoff, primarily nitrogen and phosphorus-based. In cases where fertilizer is overused, or there is heavy rainfall, nutrients can wash into nearby water bodies, leading to eutrophication. This phenomenon triggers algal blooms that deplete oxygen levels in water as the algae decomposes. This leads to the creation of hypoxic conditions dangerous to aquatic life. From my research, it is apparent that controlling the amount of fertilizer applied and having buffer spaces along waterways is more than suitable for protecting marine ecosystems from these effects.
Understanding Eutrophication and Algal Blooms
Eutrophication refers to the death of water bodies due to over-enrichment from nutrients such as nitrogen and phosphorus, which are found due to agricultural runoff and poor fertilizer management. This over-providing of resources leads to algae’s rapid growth, called algal blooms. All the information I have gathered suggests this phenomenon blocks sunlight to underwater plants and reduces the amount of oxygen when the algae dies and decomposes. As a result, all aquatic living things and fish age in these oxygen-deprived or hypoxic zones. To limit eutrophication and, in turn, protect the ecosystem, the steps I decided were managing the application of fertilizers, improving soil health, and incorporating protective measures like riparian buffers.
The Impact of Excess Nitrogen on Aquatic Life
Too much nitrogen can seriously disrupt aquatic ecosystems and promote bacteria and algal blooms. These blooms can consume oxygen at an alarming rate and create dead zones where marine life cannot exist. Further, increased nitrogen levels can change the types of species that are found in an area. It can even support those who cannot survive in areas with high biodiversity, which is troublesome for the ecosystem’s health. I also discovered that nitrogen runoff from agricultural activities contributes to the acidification of water bodies, which destroys the ecosystem by impacting shell-bearing creatures like mollusks and corals. Cover crops and reduced agrarian runoff can help deal with the acidification of waterbodies and promote better fertilizer application strategies.
Management Strategies to Protect Water Bodies
I aim first to manage water bodies with a hierarchy of processes and strategies. Lowest on the hierarchy is precision agriculture, that is, variable rate application of fertilizers that minimizes nutrient loss by ensuring the proper time, place, and amount of fertilizer is apSo calibrated nitrogen application of fertilizers with a range of 50 – 150 lbs/acre based on what the soil and crop needs along with soil tests and crop performance gives optimal results. The second strategy integrates the construction and maintenance of riparian buffer zones. These zones serve as moderate filters that reduce sediment and mineral inflow into the waterways with the minimal accepted effective width set at 35 feet. The third strategy involves integrating cover crops to reduce soil erosion and loss of nutrients, which in many studies has been shown to decrease nitrate leaching by 50% significantly. The last suggestion is monitoring the water regime during certain intervals to ensure that EPA parameters are being complied with, as nitrogen should be below 10 mg/L and dissolved oxygen levels should not drop below 5 mg/L constant. Taking all these measures adequately implements the virtual protection of the eco-system for more sustainable and protective water body systems.
What are the Environmental Consequences of Altering the Nitrogen Cycle?
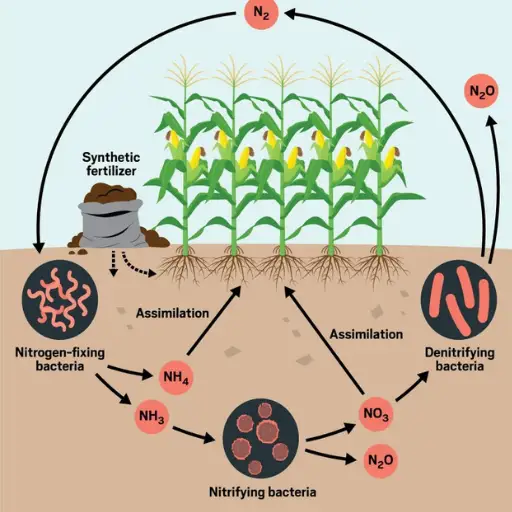
Shifts in the nitrogen cycle cause wide-ranging environmental discrepancies mainly due to the oversaturation of nitrogen species in ecosystems. The most important one is the pollution of water bodies in which the inflow containing large quantities of nitrogen, mainly in the form of nitrate, runs into fresh water and coastal regions. This leads to eutrophication, where oxygen dissolved in water is consumed, leading to hypoxic or dead zones that are hazardous to aquatic life. The atmospheric impacts include nitrous oxide N2O emissions, a significant greenhouse gas that drives climate change and stratospheric ozone destruction. Finally, excess nitrogen alters terrestrial ecosystems by distorting the plant species composition and soil quality, benefiting those tolerant to nitrogen while suppressing the ecological balance. The significance of these impacts is that they show the need to control nitrogen intake to avert environmental pollution.
Greenhouse Gas Emissions and Nitrous Oxide
My research has revealed that nitrous oxide (N₂O) is highly relevant to greenhouse gases, as its output is linked to farming activities, such as synthetic fertilizers, industrial processes, and the burning of fossil fuels. In climate change, where the focus lies on the emission of greenhouse gases, the mitigation of nitrous oxide is critical. Interestingly, excess nitrogen in soils is converted to nitrous oxide by microorganisms through processes like nitrification and denitrification. To achieve lower emissions, the N₂O problem can be solved by maximizing the amount of fertilizer applied, improving farming techniques, and developing methods for efficient nitrogen capture and reuse as recommended by other experts.
The Balance of the Nitrogen Cycle and Ecosystem Health
Based on my forensics, the nitrogen cycle is essential to the health of any ecosystem because it processes the movement of nitrogen between the atmosphere, soil, water, and organisms. However, human activities such as applying artificial fertilizers and burning fossil fuels have affected this activity and resulted in soil acidification, eutrophication of water bodies, and higher levels of greenhouse gases. According to the most current research, the nitrogen cycle can be reinstitution by implementing sustainable methods like precision farming, promoting planting cover crops, and improving wastewater treatment processes to reduce pollution and nitrogen loss.
Long-term Effects on Plant Growth and Soil Health
Multiple factors of the environment and agriculture intersect, affecting the long-term impact on plant growth and soil health. Overuse of chemical fertilizers seems to be the underlying cause of soil acidification and depletion of organic matter, which reduces soil structural integrity and microbial species richness. These processes gradually worsen the soil nutrient supply, affecting plants’ yield and productivity. However, soil health will intuitively improve if sustainable approaches like crop rotation, organic composting, and reduced tillage are adapted. Such approaches increase the nutrients available and water-holding capacity and make the plants more resilient to environmental challenges. It is essential to know that these relationships exist to make decisions focusing on maximizing agricultural production and minimizing ecological harm.
References
-
Nitrogen fertilizer effects on nitrogen cycle processes – This article discusses the impact of nitrogen fertilization on various compartments of the global nitrogen cycle. Read it here.
-
How inhibiting nitrification affects the nitrogen cycle and reduces environmental impacts of anthropogenic nitrogen input – This study examines how nitrification inhibitors impact the nitrogen cycle and reduce ecological effects caused by nitrogen fertilizers. Read it here.
-
Agriculture and the nitrogen cycle: assessing the impacts of fertilizer use on food production and the environment – This book provides an in-depth assessment of how fertilizer nitrogen is applied and its effects on the nitrogen cycle. Read it here.
Frequently Asked Questions (FAQ)
Q: What role do nitrogen fertilizers play in the nitrogen cycle?
A: Nitrogen fertilizers add nitrogen to the soil, which is essential for plant growth. However, using these fertilizers can significantly impact the nitrogen cycle by altering natural processes, such as nitrogen fixation, and increasing the amount of nitrogen compounds in the ecosystem.
Q: How does applying fertilizers affect the amount of nitrogen in the soil?
A: When fertilizers are applied, they increase the soil’s nitrogen. This can enhance plant growth but may also lead to excess nitrogen, which can cause nutrient imbalances and environmental issues like water pollution.
Q: What are the potential environmental impacts of using nitrogen fertilizers?
A: Using nitrogen fertilizers can lead to harmful algal blooms due to nutrient runoff into water bodies. It can also cause soil acidification and the release of nitrogen gas into the atmosphere, contributing to air pollution and climate change.
Q: How do fertilizers contribute to the formation of harmful algal blooms?
A: When fertilizers run off from agricultural lands, they increase the levels of nitrate and ammonium in water bodies. This nutrient enrichment promotes the rapid growth of algae, including harmful algal blooms that can disrupt aquatic ecosystems.
Q: In what ways can nitrogen fertilizers impact the nitrogen cycle?
A: Nitrogen fertilizers can impact the nitrogen cycle by altering the balance of nitrogen compounds in the soil and water. They can disrupt natural processes such as nitrogen fixation and lead to an increase in nitrogen in forms that plants may not readily use.
Q: Can using fertilizers lead to releasing nitrogen gas, and what are its effects?
A: Yes, using fertilizers can lead to the release of nitrogen gas through processes like denitrification. This release can contribute to atmospheric pollution and reduce the effectiveness of the nitrogen cycle in maintaining ecosystem balance.
Q: What measures can be taken to minimize the negative impacts of fertilizers on the nitrogen cycle?
A: To minimize adverse impacts, it is essential to use fertilizers judiciously, employing practices such as precision agriculture, crop rotation, and the use of organic fertilizers that promote the natural cycle of nitrogen without causing excess nutrient buildup.
Q: How does the addition of nitrogen fertilizers affect organic molecules from inorganic molecules?
A: Nitrogen fertilizers can facilitate the conversion of inorganic nitrogen compounds into organic molecules by providing plants with the necessary nutrients for growth and development, thereby supporting the natural nitrogen cycle.
Q: Why is the nitrogen cycle essential for maintaining ecosystem health?
A: The nitrogen cycle is a natural process essential for maintaining ecosystem health because it regulates the availability of nitrogen, a critical nutrient for plant and animal life. Balanced nitrogen cycling supports biodiversity and ecosystem productivity.






