Introduction
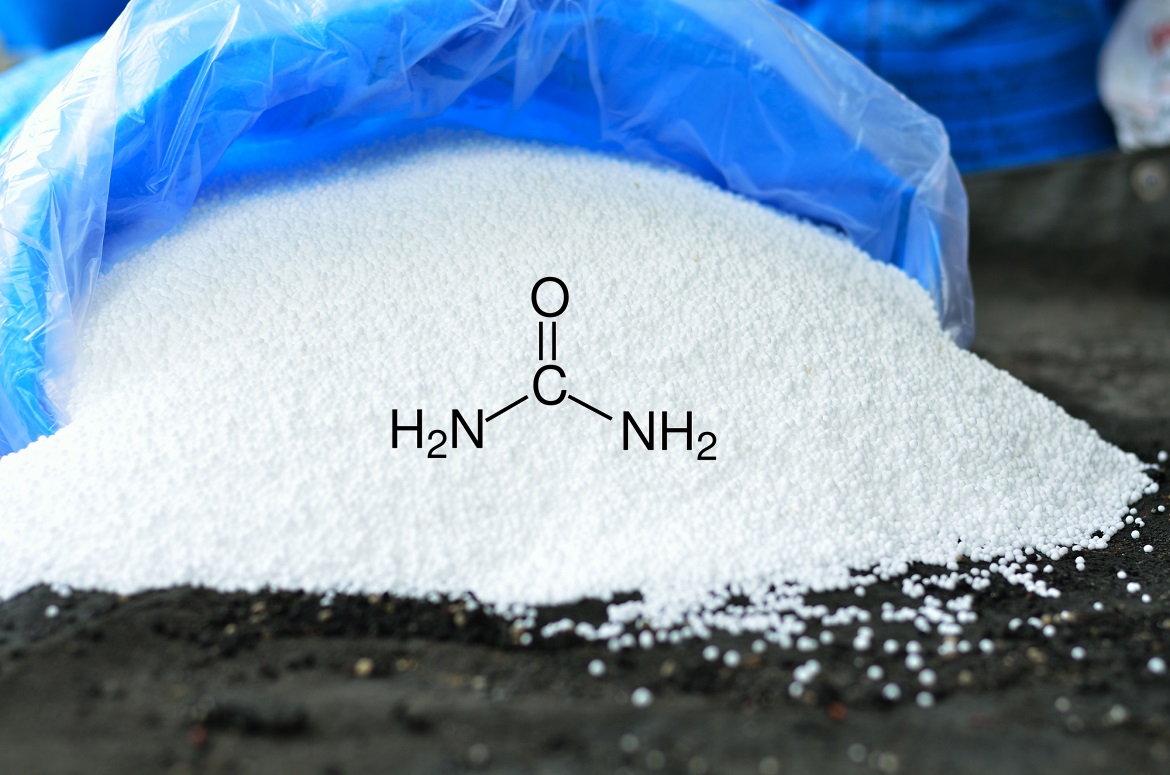
Urea fertilizer is the most popular nitrogenous fertiliser in agriculture today, often referred to as ‘the king of fertilisers’ because of its high nitrogen content and its efficiency of delivery for plant growth. Urea is the bringer of nitrogen in agricultural systems, the keystone of the food chain supply. Historically, the most common sources of nitrogen used to fertilise plants were organic resources, such as manure. But today, the majority of nitrogen fertiliser in agriculture takes the form of urea. Its chemical formula, CO(NH₂)₂, makes it an effective carrier of nitrogen that is critical to the synthesis of proteins in plants.
But to understand why urea is effective, farmers need to know the chemical formula and properties of the molecule. How urea releases nitrogen into the soil, the way it interacts with water and behaves under different environmental conditions limit its potential as a fertilizer. Without this knowledge, a farmer might be using urea in a manner that has very little impact on crops or could even be environmentally damaging.
Chemical Composition of Urea Fertilizer
One of the simplest organic compounds is also one of the most essential of agricultural science. Urea, CO(NH₂)₂, is an artificial nitrogen fertilizer consisting of carbon, oxygen and nitrogen, made of ammonia and carbon dioxide under high pressure and temperature (through what has come to be known as the Haber-Bosch process) and assembled in a hexagonal molecule. The white, crystalline product, extremely soluble in water, is a quick and effective delivery system for nitrogen in agriculture.
The industrial synthesis of this molecule through the Haber-Bosch process represents a major engineering feat. Although initially produced for ammunitions manufacture, this process paved the way for agricultural applications with a stable solid form of reactive nitrogen. Here, atmospheric nitrogen (N₂) reacts with hydrogen [mostly derived from the reaction of carbon and water in natural gas] under pressure and heat in the presence of a catalyst to form ammonia (NH₃), which in turn reacts with carbon dioxide (CO₂) to form urea. This process serves as a dramatic example of recycling at a giant scale, where otherwise we would simply waste CO₂. Moreover, this approach represents a major step in sustainable chemical processes.
Properties of Urea Fertilizer
One reason why urea dominates the fertilizer industry is not just the chemical ease with which it is manufactured, but the ease with which farmers can employ it. Key to this is the high degree of water solubility of urea, which is important because, for agriculture, the most important fertilizer component is nitrogen. When water is applied to urea, urea is quickly converted into ammonium carbonate (NH4HCO3). This salt is highly soluble in water and, therefore, plants can readily take up the nitrogen once the ammonium carbonate is washed into the field.
If the urea is applied as a solution, or if rainfall or irrigation instantly dissolves urea spread on the surface, then the nitrogen is immediately available to the plant.
Urea, then, is about 46 per cent nitrogen, making it one of the most concentrated nitrogenous fertilizers in use. Nitrogen is the third most abundant element in the Earth’s crust. Inside plants, it creates amino acids, the building blocks of proteins. It also underpins chlorophyll, the stuff that makes plants green, and allows them to capture the sun’s energy in a process called photosynthesis.
But owing to its volatility, urea can cause problems in application. Applied to the soil, urea hydrolysis, catalysed by the urease enzyme naturally existing in soils, leads to urea conversion to ammonia and carbon dioxide. With surface application, under warm and dry conditions, the ammonia may volatilise into the atmosphere, reducing the efficiency of the fertilizer as well as contributing to environmental issues such as air pollution and the greenhouse effect.
Benefits and Challenges of Using Urea Fertilizer
Benefits of Using Urea as a Fertilizer
Urea offers numerous benefits that make it a staple in agricultural practices worldwide:
Highest N content: 46 per cent The nitrogen content in urea is quite high – it is one of the most concentrated fertilizer in the world. This high level of nitrogen in urea encourages vigorous growth in the plants. It can lead to a staggering rise in the yield of the crop if applied in the right way.
Cost-Effectiveness: It’s the usual story: relatively cheaper to produce and purchase than other nitrogenous fertilizer. This factor alone attracts those with slightly (or highly) limited budgets.
Ease of application: There are three different forms of urea, which are pellets, granules and solutions. Additionally, there are different application methods available. So, applications can be made for broadcasting or side-dressing, but also through drip irrigation setups, depending on the agricultural needs.
Challenges Associated with Urea Fertilizer
Despite its benefits, urea also presents several challenges that require careful management:
Volatilisation potential: If urea is not incorporated into the soil soon after application, it can be transformed into gaseous ammonia and significant nitrogen losses will occur, both decreasing N efficiency and increasing environmental pollution.
Environmental pollution: Excessive or inappropriate urea use can lead to nitrate leaching into water bodies, causing eutrophication that can impair water quality and harm aquatic life. Ammonia in urea is also volatilised into the atmosphere, contributing to greenhouse gas emissions.
Urea depends on specific soil conditions For urea to work it needs to be hydrolysed into plant-usable ammonium and nitrate. This depends on soil conditions: the warmer the better; the moister the better, but at a certain threshold the water can act like concrete, rendering the nitrogen useless. So in very dry or very cold conditions the urea treatment is worst.
With this knowledge, farmers can better exploit the advantages of urea while minimising its possible negative effects on the environment.
Application Techniques for Urea Fertilizer
The most effective use of urea fertilizer on croplands occurs only when farmers apply the fertilizer with best practice matching crop needs and local soil conditions, and minimising losses including volatilisation and leaching. Here’s how to do it.
Best Practices for Applying Urea
Bring Urea into the Soil: Urea application should be followed by its quick incorporation in the soil to minimise volatilisation losses. Rapid incorporation can be achieved by tilling or by irrigating immediately after application. By doing so, urea gets converted to ammonium ions which are more readily absorbed by the plant roots.
Timing of application: It is important for more urea to be taken up by crops. The best time to apply urea to crops is soon before rainfall or irrigation so urea can dissolve and nitrogen loss can be minimised. For many crops, applying urea early after germination when crops are growing very vigorously improves the efficiency of nitrogen uptake.
Urease Inhibitors: Urease inhibitors reduce the rate of conversion of urea to ammonia. These are chemicals that temporarily deactivate enzyme urease, the enzyme that catalyses urea hydrolysis. Applying them reduces the risk of volatilisation of ammonia.
The Role of Timing and Soil Conditions
Water to soil moisture: Urea should be applied when the soil is moist, and there is an expectation for the soils to be moist (from rainfall or supplemental irrigation) to quickly dissolve the urea granules into a dissolved solution before they can sit on the surface and allow nitrogen to volatilise into the air, wasting your money.
Soil Temperature: Urea’s hydrolysis rate decreases with cooler soil temperatures, which reduces volatilisation losses, but very cold temperatures slow the urea down so much that it might disrupt its ability to be absorbed by roots.
Applying these practices helps to ensure that urea fertilizer is applied to soils at the right time, and under the right conditions, and given to plants in the right manner so that they have enough nitrogen to thrive while minimising loss to the environment.
Conclusion
The exploration of the urea fertilizer formula in plant cultivation along with its usages demonstrates the scientific basis of urea, made with the chemical formula CO(NH₂)₂, and its practical usage and applications to enrich the plant growth potential and crop yields.
We’ve covered its manufacture and chemistry, and how the combination of that unique ability to be both readily soluble and highly nitrogen-rich makes urea both potent and highly particular in its environmental sensitivity. Proper timing of application, techniques for soil incorporation, and the use of urease inhibitors all contribute to the most effective uses and ultimately the most sustainable applications of urea fertilizer.
Given that both the requirement of agriculture and our understanding of the environment evolves over time, we must have an open mind about how urea and similar fertilizers can be used in an increasingly efficient manner. Farmers and agriculturists are always encouraged to learn and update themselves so that urea fertilizers can always remain as a boon to agricultural productivity.
In the future, the better adaptation and scientific research will help in making the best use of urea in agriculture and getting the best out of its abilities and potential.
Here are some scholarly references on urea fertilizer:
- Modified Urea Fertilizers and Their Effects on Improving Nitrogen Use Efficiency (NUE): This article discusses the chemical properties of urea (CO(NH₂)₂), its application as a nitrogenous fertilizer, and the challenges related to nitrogen loss through volatilization and leaching. It also explores the potential benefits of modified urea formulations that aim to improve nitrogen use efficiency by controlling the release of nitrogen, thereby reducing environmental impact.
- Urea Formula, Properties & Application: This resource provides a detailed overview of urea, also known as carbamide, which has the formula CO(NH₂)₂. It covers urea’s discovery, synthesis, and various applications, particularly in agriculture as a high-nitrogen release fertilizer. It also discusses the environmental impacts of urea use, such as potential contributions to water pollution if not managed properly.







